October 8, 2021
 ~5m
~5mProsthetic valve choice and mode of intervention […]
The latest ESC/EACTS Guidelines: A focus on valve durability, available evidence and valve replacement innovation
Prosthetic valve choice and mode of intervention for aortic valve replacement in younger patients have been controversial because of concerns about long-term durability. The 2021 update to the ESC/EACTS guidelines for the management of valvular heart disease makes recommendations for patients with severe aortic stenosis, based on available evidence and
valve replacement innovation.1
In patients with severe aortic stenosis, age is now a key determining factor in the initial
choice between SAVR or TAVI. This change simplifies the initial patient treatment pathway
and preferred mode of intervention for patients with severe aortic stenosis.
The guidelines emphasise that while the durability of surgical bioprosthetic valves
beyond 10 years is well established, evidence supporting the durability of TAVI valves is
more limited.

As valve durability is a key consideration in younger patients (<75 years) at low surgical risk, SAVR (if feasible) is the preferred treatment option. SAVR is also recommended for patients who are unsuitable for transfemoral TAVI and operable.
The guidelines recommend TAVI for older patients (≥75 years) and those who are inoperable or high risk for surgery (particularly if feasible via the transfemoral approach).
SAVR or TAVI are recommended for remaining patients according to individual
clinical, anatomical and procedural characteristics.
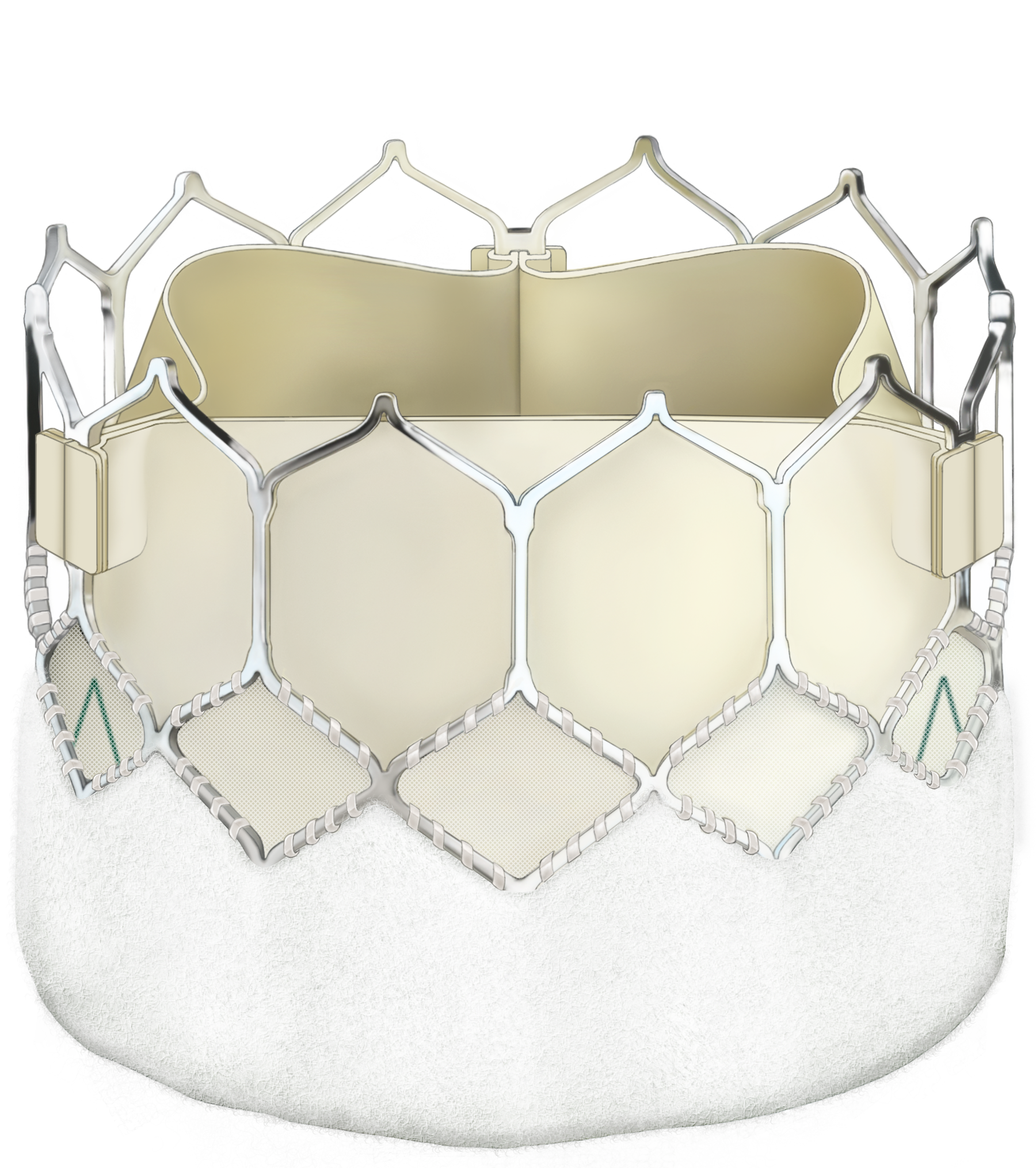
Prosthesis selection: Tissue or mechanical valve?
If the Heart Team and patient agree that SAVR is the way forward, the choice between mechanical and biological valves comes to the fore. Key factors for the Heart Team to consider are:
- The patient’s life expectancy
- Lifestyle and environmental factors
- Bleeding and thromboembolic risk related to anticoagulation
- Potential for surgical or transcatheter reintervention
- Informed patient choice
The evidence continues to support the use of aortic bioprostheses for patients over 65 years of age, but just how durable are bioprostheses?
Latest recommendations are supported by real-world evidence and consistent clinical outcomes
Several large, long-term studies have demonstrated the excellent long-term durability of the Carpentier-Edwards PERIMOUNT aortic valve.2–9
Overall expected valve durability of the PERIMOUNT aortic valve is 19.7 years.2-4
- Broken down by age, durability was
- 17.6 years for patients aged ≤60 years
- 19 years for patients aged 50–65 years
- 22.1 years for patients aged 60–70 years
- Twenty-year probability of explant due to SVD across all age groups is 5.4%7
- Younger age at implantation is associated with a higher risk of structural valve deterioration,2,6-9 but durability is good even in these younger patients2,6-9
Recommended resources
References
1. Vahanian A, Beyersdorf F, Praz F et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021:
2. Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P et al. Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015; 99: 831–7.
3. Bourguignon T, El Khoury R, Candolfi P et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 60 or younger. Ann Thorac Surg. 2015; 100: 853–9.
4. Bourguignon T, Lhommet P, El Khoury R et al. Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 50–65 years. Eur J Cardio-Thorac Surg. 2016; 49: 1462–8.
5. Forcillo J, El Hamamsy I, Stevens LM et al. The Perimount valve in the aortic position: Twenty-year experience with patients under 60 years old. Ann Thorac Surg. 2014; 97: 1526–32.
6. Forcillo J, Pellerin M, Perrault LP et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg. 2013; 96: 486–93.
7. Johnston DR, Soltesz EG, Vakil N et al. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann Thorac Surg. 2015; 99: 1239–47.
8. McClure RS, Narayanasamy N, Wiegerinck E et al. Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg. 2010; 89: 1410–6.
9. Minakata K, Tanaka S, Okawa Y et al. Long-term outcome of the Carpentier-Edwards Pericardial valve in the aortic position in Japanese Patients. Circ J. 2014; 78: 882–9.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI, PERIMOUNT, RESILIA and SAPIEN are trademarks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2949 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.