Biological versus mechanical prostheses for aortic valve replacement
Rodríguez-Caulo, Emiliano A., et al., Journal of Thoracic and Cardiovascular Surgery (2021) DOI:10.1016/j.jtcvs.2021.01.118
Aim
Evaluate long-term survival and major adverse events in patients aged 50 to 65 years with a primary isolated AVR, with a bioprosthesis or mechanical valve, because of severe AS
Methods
- National observational study with all consecutive patients aged 50-65 who underwent AVR because of severe isolated AS between 2000 and 2018 in 27 hospitals in Spain
- In total, 5215 patients were included in the study (21% were bioprostheses)
- Among bioprostheses, 46.8% were Carpentier-Edwards, 19.4% Mitroflow, 4.2% Mosaic and 4.1% Trifecta
- 2:1 propensity matching analysis (1822 Mech and 911 Bio) with a mean follow-up of 8.1 years
Results
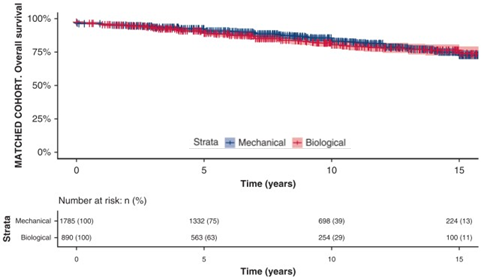
- In the matched cohort, long-term survival differences were not observed (cf. figure)
- Overall survival after Mech valves at 5, 10, 15 and 18 years of follow-up were 91%, 83%, 73% and 62% vs. 89%, 81%, 74% and 72% for bioprostheses, respectively
- Minor differences in survival were found in patients aged 50-55 years (P = 0.11)
- No significant differences in the composite of stroke, bleeding and reintervention (P = 0.484)
- Major bleeding (P = 0.002) and strokes (P = 0.095) had a higher frequency in the Mech valve group
- Rates of reoperation were significantly higher in the bioprosthesis valve group (P < 0.001)
- In a 1:1 propensity matching, the risk of reintervention in the Bio group decreased for patients who underwent AVR between 2009 and 2018 compared with 2000 to 2008
Conclusions
- Bioprostheses seem a reasonable choice for patients aged 50 to 65 years in Spain, particularly for those older than 55 years, because of the long-term survival and the lower-risk related, especially, to major bleeding compared with mechanical prostheses
- However, the higher risk of reoperation with bioprostheses should be considered and discussed with the patient to make the best-informed decision
Key talking points
Are these outcomes aligned with guideline recommendations? – Current ESC/EACTS guidelines consider mechanical and bioprosthetic valves as reasonable in patients aged 60 to 65 years, whereas AHA/ACC guidelines consider both reasonable in 50 to 65 y.o. patients. Therefore, findings from this national observational study with patients that underwent isolated AVR seem to be more aligned with the latest American guidelines.
Why did the risk of reintervention decrease in patients who underwent Bio AVR between 2009 and 2018? – The authors point to improved outcomes after AVR for both mech and bio prostheses after 2009. In the unadjusted cohort, there was a significant decrease in stroke, major bleeding and reintervention. In the bio group, the rate of reoperation because of SVD diminished from 7.6% to 4% after 2009, which is related to the durability of bioprostheses. Improvements in the bio group may be linked to the incorporation of newer anticalcification properties.
What is the mortality risk associated with prosthesis reintervention? – Today, mortality risk associated with prosthesis reintervention has been minimized, and most recent bioprostheses allow for “valve-in-valve” procedures for patients at high surgical risk.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2029 V1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com





 ~5m
~5m