With the publication of the 2021 update […]
Mechanical versus bioprosthetic valves for severe aortic stenosis
With the publication of the 2021 update to ESC/EACTS guidelines for the management of valvular heart disease, new guidance should be considered when caring for patients with severe aortic stenosis.1
Three key recommendations stand out:

Early intervention is recommended for asymptomatic patients with a LVEF below 55% without another cause

An emphasis on Heart Team collaboration to provide the optimal treatment while informing and involving patients in their decision-making
A clear age delineation that patients younger than 75 years of age with severe aortic stenosis receive SAVR, and patients older receive TAVI

It is established that the durability of modern surgical bioprosthetic valves is over 10 years,2 and in recent years there has been a trend towards a greater use of bioprosthetic valves.3 On the other hand, mechanical valves continue to be associated with bleeding risks because of the need for lifelong anticoagulation therapy, leading to lifestyle changes.4-6
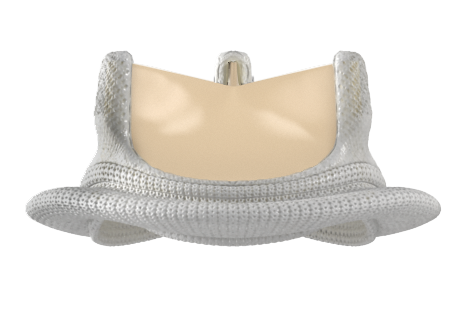
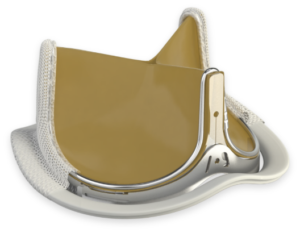
INSPIRIS RESILIA aortic valve
Another study found that the INSPIRIS RESILIA valve reduced the length of hospital stay compared with mechanical valves (6.6 days versus 10.3 days).11 Selecting the right treatment for the patient requires many considerations, most importantly the desire of the informed patient.
Additionally, the Heart Team needs to consider the age, estimated life expectancy, comorbidities, and anatomical and procedural characteristics of the patient; relative risks and long-term outcomes associated with SAVR or TAVI; prosthetic heart valve durability; feasibility of transfemoral TAVI; and local experience and outcome data to achieve optimal outcomes for patients.1
Recommended resources
References
1. Vahanian A, Beyersdorf F, Praz F et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur Heart J. 2021: doi:10.1093/eurheartj/ehab395.
2. Johnston DR, Soltesz EG, Vakil N et al. Long-term durability of bioprosthetic aortic valves: Implications from 12,569 implants. Ann Thorac Surg. 2015; 99: 1239–47
3. Bartus K, Sadowski J, Litwinowicz R et al. Changing trends in aortic valve procedures over the past ten years—from mechanical prosthesis via stented bioprosthesis to TAVI procedures—analysis of 50,846 aortic valve cases based on a Polish National Cardiac Surgery Database. J Thorac Dis. 2019; 11: 2340–9.
4. Head SJ, Çelik M, Kappetein AP. Mechanical versus bioprosthetic aortic valve replacement. Eur Heart J. 2017; 38: 2183–91.
5. Reul RM, Ramchandani MK, Reardon MJ. Transcatheter aortic valve-in-valve procedure in patients with bioprosthetic structural valve deterioration. Methodist DeBakey Cardiovasc J. 2017; 13: 132.
6. Swinkels B, Ten Berg J, Kelder J et al. What can we learn from the past by means of very long-term follow-up after aortic valve replacement? J Clin Med. 2021; 10: 3925.
7. Bourguignon T, Bouquiaux-Stablo A-L, Candolfi P et al.Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg. 2015; 99: 831–7.
8. Forcillo J, Pellerin M, Perrault LP et al. Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg. 2013; 96: 486–93.
9. Bartus K, Litwinowicz R, Bilewska A et al. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur J Cardio-Thorac Surg. 2021; 59: 434–41.
10. Bavaria J, Griffith B, Heimansohn DA et al. Five-year outcomes of the COMMENCE trial investigating aortic valve replacement with a novel tissue bioprosthesis. Presented at the Society of Thoracic Surgeons (STS) Annual Meeting 2021.
11. Meuris B. Innovation in anticalcification technology in heart valves leads to lower hospital stay in adults undergoing aortic valve replacement. Oral Presentation at HTAi 2021 virtual congress.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, COMMENCE, INSPIRIS, INSPIRIS RESILIA, PERIMOUNT, and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2950 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.





 ~5m
~5m