The recently updated ESC/EACTS Guidelines contain two […]
Promising results for bioprosthetic SAVR in patients under 60 years old
The recently updated ESC/EACTS Guidelines contain two key changes in the recommendations for prosthetic valve selection:1
- A new Class IIb recommendation for bioprostheses in patients already on long-term NOACs
- An upgraded Class I recommendation for bioprostheses in patients for whom good-quality anticoagulation is unlikely or contraindicated, and in patients whose life-expectancy is lower than the presumed durability of the valve
Importantly, the desire of the informed patient remains central to prosthetic valve selection. Valve durability in young patients is an important consideration, but long-term data on bioprosthetic SAVR have been limited.
INDURE registry
INDURE is a prospective, open-label, multicentre registry that is tackling this issue head-on. INDURE has enrolled over 400 patients aged up to 60 years who are undergoing SAVR with the INSPIRIS RESILIA valve in 21 sites across Europe and Canada. Patients are being followed up for five years, with echocardiograms analysed by Echo Core Laboratory at years one and five.2,3
One-year results from the first 435 patients were reported at the 2021 EACTS Annual Meeting. Younger patients (up to 50 years old) in the registry were more likely to have a bicuspid aortic valve or aortic valve regurgitation at baseline than patients aged 51–60 years, but were less likely to have aortic stenosis, hypertension or diabetes.3
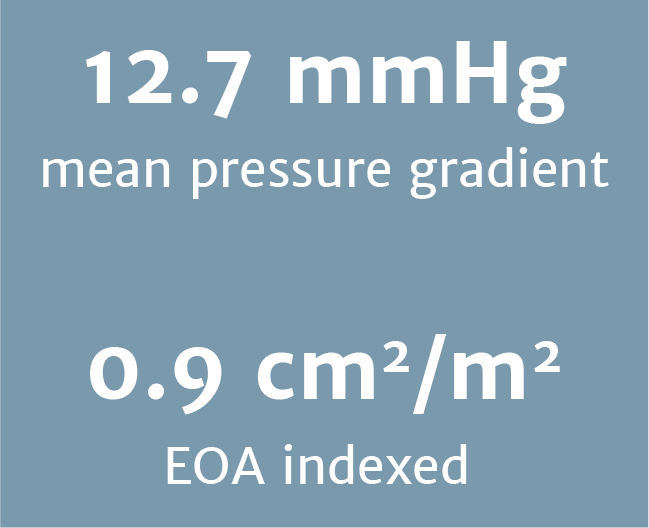
Excellent haemodynamic outcomes at 1 year
The excellent haemodynamic outcomes were comparable in the younger and older patient groups.
Plus, preliminary safety outcomes demonstrated low all-cause mortality and no confirmed cases of valve-related mortality up to one year. Rates of endocarditis and stroke were low (<1%). There were no cases of stage 3 SVD.
The INDURE registry will continue to provide clinical evidence on the use of the INSPIRIS RESILIA valve in young patients for the next 5 years.
References
1. Vahanian A, Beyersdorf F, Praz F et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease. Eur J Cardiothorac Surg. 2021.
2. Meuris B, Borger MA, Bourguignon T et al. Durability of bioprosthetic aortic valves in patients under the age of 60 years – rationale and design of the international INDURE registry. J Cardiothorac Surg. 2020; 15: 119.
3. De Paulis R. Surgical aortic valve replacement in patients under 60 years old: A prospective, multicentre real-world registry in Europe and Canada. EACTS 2021.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the instructions for Use (consult eifi.edwards.com where applicable)
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, INSPIRIS, and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-3060 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.




 ~5m
~5m














