At 5 years, COMMENCE results indicate a favourable safety profile and stable haemodynamic performance of a bioprosthetic valve with RESILIA tissue. Ten-year data in an extended follow-up cohort and the RESILIENCE trial with 11-year follow-up are forthcoming.
Suggested Posts for you
Durability: An even more important factor in valve selection
The 2021 update of the ESC/EACTS Guidelines […]
Durability: An even more important factor in valve selection
The 2021 update of the ESC/EACTS Guidelines for the management of valvular heart disease, recommends treating patients with severe aortic stenosis younger than 75 years and low risk with surgical aortic valve replacement (SAVR), simplifying the initial patient treatment pathway and preferred mode of intervention. The implication is that valve durability is now even more important because of younger patients’ greater longevity.
Edwards Lifesciences has two ongoing trials, COMMENCE and EU Feasibility, that are evaluating the longevity of a bioprosthetic valve with novel RESILIA tissue. Both trials have demonstrated a favourable safety profile and good haemodynamic performance at 5 years, with additional follow-up to be conducted up to 10 years.1,2

The COMMENCE trial
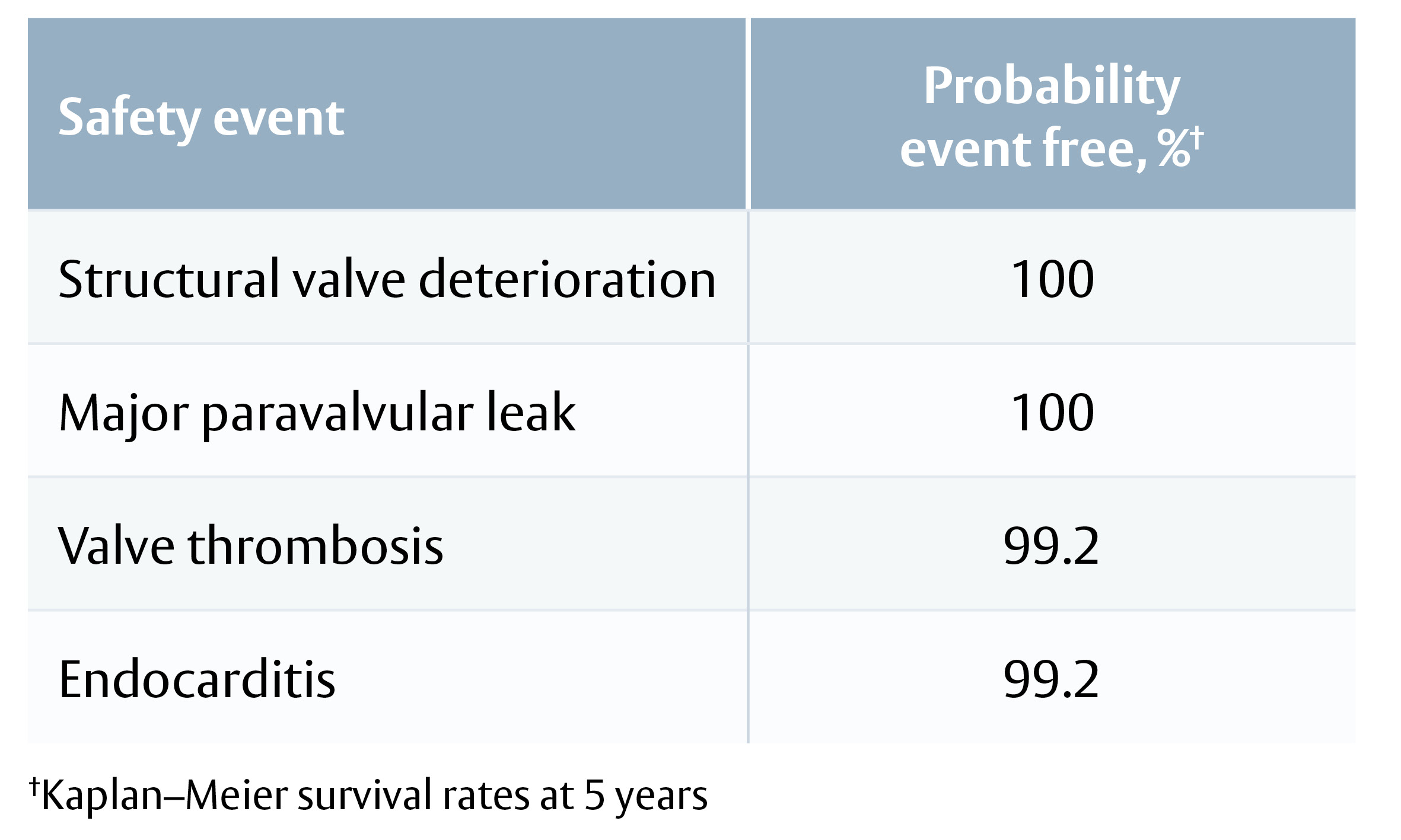
Safety endpoints, probability event-free:
COMMENCE is an FDA pivotal trial designed to evaluate the safety and effectiveness of the INSPIRIS bioprosthetic aortic valve with the novel RESILIA tissue.1 The 5-year data are now available:
· All-cause mortality 89.2%
· Major paravalvular leak 99.5%
· Endocarditis 97.8%
Improved haemodynamic performance from baseline
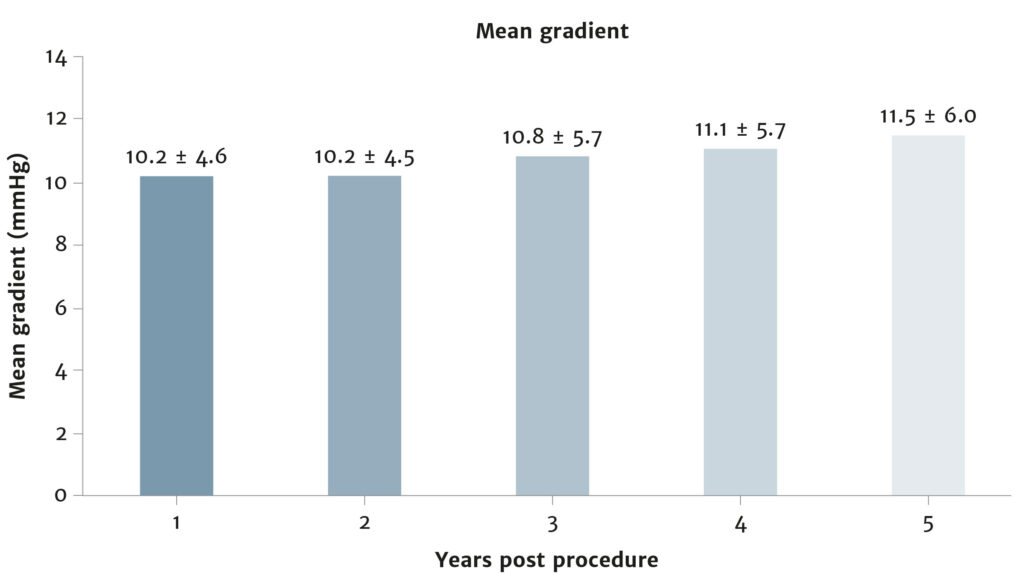
The absence of structural valve deterioration through 5 years* and the presence of stable gradients, plus freedom from regurgitation support durability over the observational period.
*One case of structural valve deterioration was diagnosed at postoperative Day 1,848.
The RESILIA EU Feasibility study
The RESILIA EU Feasibility study has investigated the safety and performance in aortic valve replacement patients of the bioprosthesis with the novel RESILIA tissue at 5 years.2 Here, we summarise the results at 5 years:
Stable haemodynamic performance:
Mean gradient was 14.8 ± 7.6 mmHg
Average effective orifice area was 1.4 ± 0.5 cm2
Zero structural valve deterioration events and stable transvalvular gradients were observed at 5 years.
At 5-years, results from the EU Feasibility study represent the longest follow-up of aortic valve replacement patients with RESILIA tissue and demonstrate excellent haemodynamic performance and safety outcomes.1
Recommended resources
https://edwardseducation.com/edwardsmasters/the-latest-tissue-valve-technology-responding-to-the-unmet-needs-in-avr/
References
1. Bavaria J, Griffith B, Heimansohn DA et al. Five-year outcomes of the COMMENCE trial investigating aortic valve replacement with a novel tissue bioprosthesis. Presented at the Society of Thoracic Surgeons (STS) Annual Meeting, January 29-31, 2021.
2. Bartus K, Litwinowicz R, Bilewska A et al. Final 5-year outcomes following aortic valve replacement with a RESILIA™ tissue bioprosthesis. Eur J Cardiothorac Surg 2020; 59: 434–41.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, INSPIRIS, INSPIRIS RESILIA, and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2021 Edwards Lifesciences Corporation. All rights reserved. PP–EU-2948 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

Welcome to
New Edwards Masters
Your European Educational Platform in Heart Valve Surgery,
now fully customized to your needs and interests. Your educational platform
in heart valve surgery.
now fully customized to your needs and interests. Your educational platform
in heart valve surgery.
New to Edwards Masters? Sign Up!




 ~5m
~5m














