Reintervention after aortic valve replacement: Comparison of three aortic bioprostheses
Keywords: aortic valve replacement; pericardial bioprostheses; reintervention
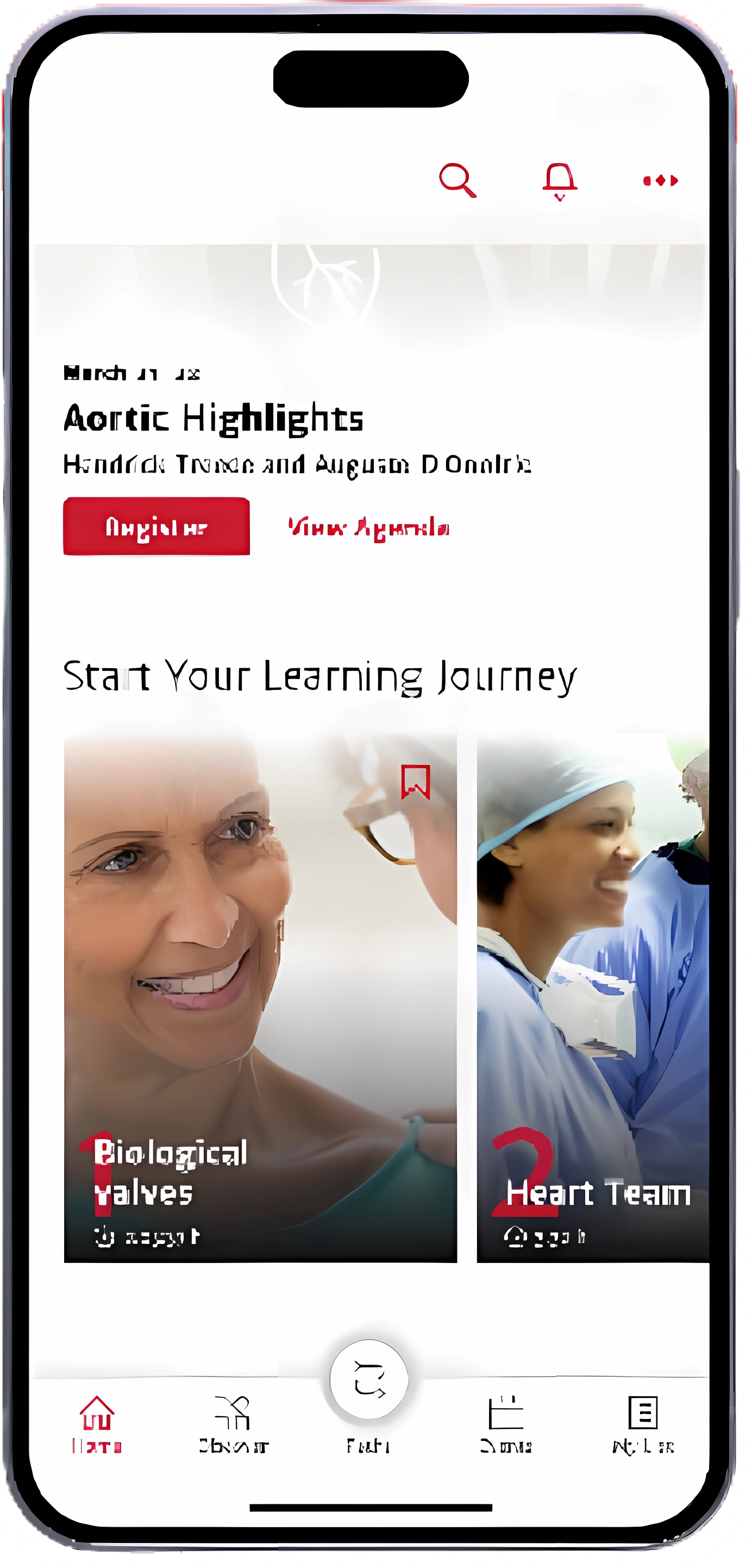
The use of pericardial bioprostheses in aortic valve replacement (AVR) is highly popular due to the lack of need for lifelong anticoagulation. Despite this, few studies have investigated the durability, rates of structural valve degeneration and valve-related events in newer bioprostheses.
In this article Lam et al. present key results from a single-centre, retrospective study comparing three different aortic bioprosthetic valves . The authors assessed rates and causes of reinterventions in patients who underwent AVR with the Trifecta, Mitroflow and Carpentier-Edwards PERIMOUNT Magna Ease valves.
Follow the link below for the latest research in aortic valve replacement.
Click here to access the full publication
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Magna, Magna Ease, PERI, PERIMOUNT, and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.




 ~5m
~5m