December 6, 2022
 ~5m
~5mCibin G, D’Onofrio A, Lombardi V, Bergonzoni […]
Propensity score analysis of stented vs rapid deployment aortic bioprostheses in patients with small aortic annulus
Cibin G, D’Onofrio A, Lombardi V, Bergonzoni E, Italiano E, Gastino E, Evangelista G, Cao I and Gerosa G.
Presented at 36th European Association for Cardio-Thoracic Surgery Annual Meeting, 2022.
Key points:
- In patients with a small aortic annulus, the EDWARDS INTUITY valve was associated with reduced implantation times and improved haemodynamic results compared with implantation of the Carpentier-Edwards PERIMOUNT Magna Ease valve.
- Perioperative outcomes, survival and rehospitalisation rates were similar for both devices.
Background information
- Rapid deployment aortic bioprostheses (RDBs) have been developed to reduce operating times and allow for minimally invasive procedures.
- RDBs have shown excellent performance in haemodynamics studies.
Aims
- To compare early and medium-term outcomes for RBDs versus conventional stented valves in patients with small aortic annulus.
Type of study
- A retrospective single-centre study.
Endpoints
- Endpoints included operating times, haemodynamics, mortality and rehospitalisation.
Methods
- The study included patients who underwent isolated or combined surgical aortic valve replacement with a size 19 or 21mm EDWARDS INTUITY valve (RDB) or PERIMOUNT Magna Ease (conventional stented) valve.
- Propensity score matching was used to control for baseline differences between study groups.
- Follow-up visits were either scheduled on-site examinations that included echocardiography, or telephone interviews.
Results
Patients
- The study considered 666 patients, 220 of whom were included in the propensity-score matched analysis population.
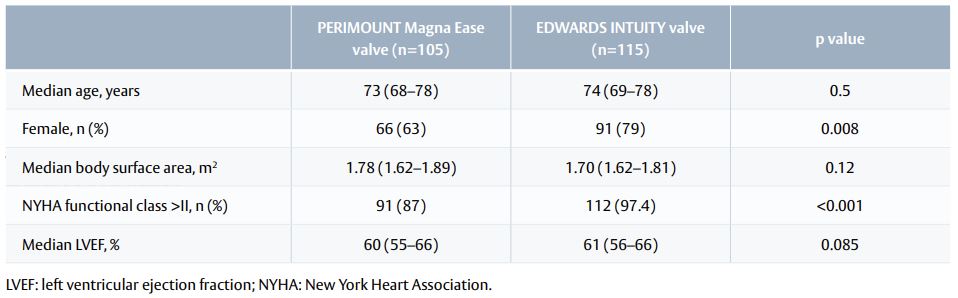
- Of these 220 patients, 115 (52%) received an EDWARDS INTUITY valve and 105 (48%) received a PERIMOUNT Magna Ease valve (Table 1).
- The median follow-up time was 755 days.
Operating times
- The EDWARDS INTUITY valve was associated with significantly reduced cardiopulmonary bypass time vs the PERIMOUNT Magna Ease valve (median [95% CI]: 104 [76–129] min vs 132 [109–180] min; p<0.001).
- The aortic cross-clamp time was also significantly shorter with the EDWARDS INTUITY valve than with the PERIMOUNT Magna Ease valve (74 [54–98] min vs 100 [86–134] min; p<0.001).
Table 1. Baseline characteristics in the propensity-score matched population.
Haemodynamics
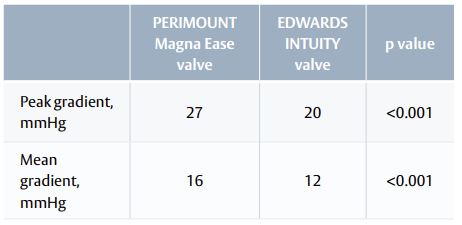
- Patients with an EDWARDS INTUITY valve had better haemodynamic outcomes than patients with a PERIMOUNT Magna Ease valve (Table 2).
Table 2. Haemodynamics
Survival and rehospitalisation
- Survival and rehospitalisation rates were similar across both study groups (p=0.761 and p=0.570, respectively).
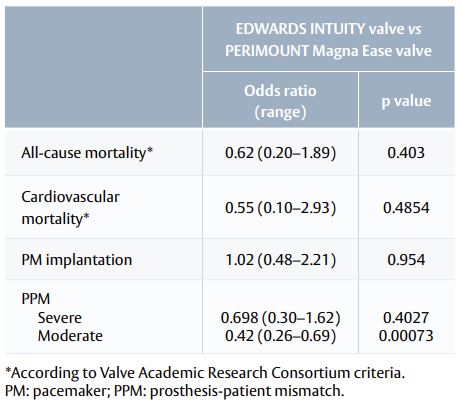
- Adverse events outcomes were also similar, except for the occurrence of moderate prosthesis-patient mismatch (PPM; Table 3).
Conclusion
- In patients with a small aortic annulus, the EDWARDS INTUITY valve allowed for shorter operating times and improved haemodynamic function versus the PERIMOUNT Magna Ease valve. Perioperative outcomes, medium-term survival and rehospitalisation rates were similar across study groups.
Table 3. Adverse events with the EDWARDS INTUITY valve vs the PERIMOUNT Magna Ease valve.
This document is a summary of the Cibin G et al. presentation, as presented at the EACTS congress, and covers key information including aim, type of study, methods, results, limitations and conclusions.
The full publication is available at:
Abbreviations

Important safety information: Use of the EDWARDS INTUITY Elite valve system may be associated with new or worsened conduction disturbances, which may require a permanent cardiac pacemaker implant (PPI). The rate of PPI for the EDWARDS INTUITY Elite valve is within the range reported in the literature for various rapid deployment valves, but higher than that reported for surgical aortic valves. Physicians should assess the benefits and risks of the EDWARDS INTUITY Elite valve prior to implantation. See instructions for use for additional information.
For professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards devices placed on the European market meeting the essential requirements referred to in Article 3 of the Medical Device Directive 93/42/EEC bear the CE marking of conformity.
Edwards, Edwards Lifesciences, the stylized E logo, and EDWARDS INTUITY are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-3975 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.