December 6, 2022
 ~5m
~5mFrancica A, Tonelli F, San Biagio L, […]
PERIMOUNT Magna Ease vs INSPIRIS RESILIA valve in patients below 70 years of age: a propensity-matched analysis of the haemodynamic performances
Francica A, Tonelli F, San Biagio L, Rossetti C, Tropea I, Luciani GB, Faggian G and Onorati F.
Presented at 36th European Association for Cardio-Thoracic Surgery Annual Meeting, 2022.
Key points:
- The safety and efficacy outcomes of the new INSPIRIS RESILIA valve in patients younger than 70 years were comparable to the established Carpentier-Edwards PERIMOUNT Magna Ease valve at mid-term.
- At Year 3, haemodynamic outcomes remained stable and were similar between INSPIRIS RESILIA valve and the comparator.
Background information
- The third-generation PERIMOUNT Magna Ease valve is the gold standard bioprosthesis for patients of all ages.
- The INSPIRIS RESILIA valve is a new bovine pericardial valve, and data on its use in patients aged ≤70 years are currently limited.
Aims
- To compare the haemodynamic performance of the INSPIRIS RESILIA valve vs the PERIMOUNT Magna Ease valve in aortic position in patients younger than 70 years who had undergone aortic valve replacement surgery.
Type of study
- A propensity score-matched analysis.
Endpoints
- The endpoint was haemodynamic performance, including mean pressure gradient, peak velocity (Vmax) and the left ventricular end-diastolic volume (LVEDV) at Year 1.
Methods
- The study included patients aged <70 years who received an INSPIRIS RESILIA valve between 2017 and 2022 or a PERIMOUNT Magna Ease valve between 2010 and 2012.
- Propensity score-matching was used to account for differences in baseline characteristics, including age, diabetes, hypertension, dyslipidaemia, atrial fibrillation, bicuspid valve and moderate–severe aortic regurgitation.
- Patients underwent follow-up examination with echocardiography 1 year after surgery.
Results
Patients
- The study included 238 patients with PERIMOUNT Magna Ease valve and 192 with INSPIRIS RESILIA valve; 122 of each group were included in the propensity score-matched analysis.
- In the propensity-matched cohort, the mean age was 7 ± 11.1 years in the PERIMOUNT Magna Ease group and 57.0 ± 9.1 years in the INSPIRIS RESILIA group (p=0.098); 19.7% of patients in the PERIMOUNT Magna Ease group and 25.4% of patients in the INSPIRIS RESILIA group were female (p=0.284). EuroSCORE II was 2.7 ± 2.4% in both groups (p=0.788).
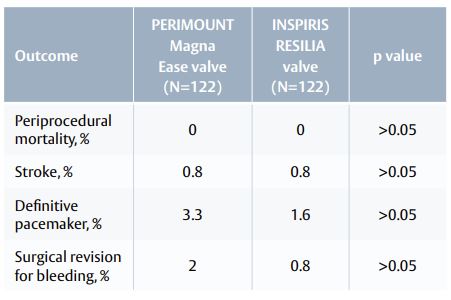
- Periprocedural outcomes were similar and not statistically significant between INSPIRIS RESILIA and PERIMOUNT Magna Ease valves.
Table 1. Periprocedural outcomes
Haemodynamic performance
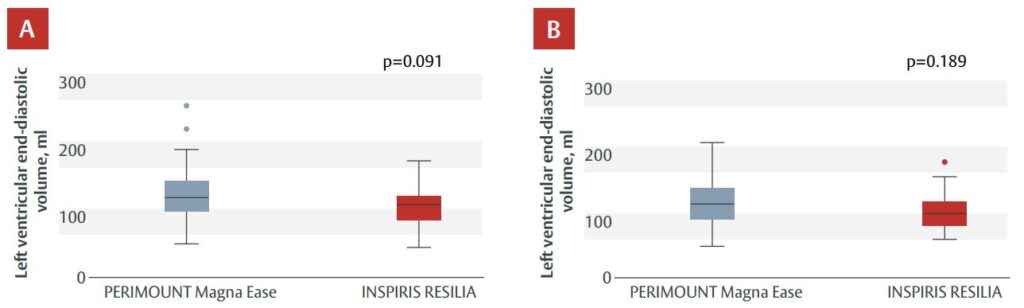
- At 1 and 3 years, the differences in mean pressure gradient, Vmax or LVEDV (Figure 1) with the INSPIRIS RESILIA valve vs the Magna Ease valve did not reach statistical significance. Mean gradients and peak velocity was also similar between groups across different valve sizes (19–21 mm, 23–25 mm and 27–29 mm).
- At 3 years, there was no reports of structural valve deterioration in the INSPIRIS RESILIA valves; four cases were reported in the PERIMOUNT Magna Ease valve, with 2 patients experiencing moderate–severe aortic stenosis and two patients reporting moderate–severe regurgitation.
Conclusion
The safety and efficacy outcomes of the new INSPIRIS RESILIA valve in patients younger than 70 years were comparable to the excellent outcomes in the established PERIMOUNT Magna Ease valve. Both valves also showed stable and comparable haemodynamics at short- to mid-term follow-up. These results may even suggest improved long-term outcomes in younger patients receiving the INSPIRIS RESILIA valve, however, more studies are needed to establish the potential advantages of this valve.
This document is a summary of the Francica A et al. presentation and covers key information including aim, type of study, methods, results and conclusions.
The full publication is available at:
Abbreviations:

Figure 1. LVEDV at (A) Year 1 and (B) Year 3
No clinical data are available that evaluate the long term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI, PERIMOUNT, PERIMOUNT Magna and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-5308 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere