December 8, 2022
 ~5m
~5mMontero Cruces L, Carnero Alcázar M, Perez […]
Five years hemodynamic performance of three aortic bioprostheses. A randomized clinical trial
Montero Cruces L, Carnero Alcázar M, Perez Camargo D, Cobiella Carnicer J, Campelos Fernandez P, Reguillo Lacruz FJ and Maroto Castellanos LC.
Presented at 36th European Association for Cardio-Thoracic Surgery Annual Meeting, 2022.
Key points:
- In the BEST-VALVE randomised clinical trial, the Carpentier-Edwards PERIMOUNT Magna Ease valve achieved the best haemodynamic performance 5 years after surgery, compared with the Crown PRT and Trifecta valves.
Background information
- The PERIMOUNT Magna Ease, Crown PRT and Trifecta pericardial valves are used worldwide in aortic valve replacement surgery.
- High-quality data comparing the haemodynamic performance of the three bioprostheses are sparse.
Aims
- To compare the medium-term haemodynamic performance of the PERIMOUNT Magna Ease, Crown PRT and Trifecta biprostheses.
Type of study
- Longitudinal, single-centre, observer-blind, randomised Phase IV study. (European database of clinical trials: 2018-001658-57.)
Endpoints
- The primary endpoint was the haemodynamic performance (mean and peak aortic gradients, peak aortic velocity and effective orifice area) 5 years post-surgery.
- Secondary endpoints included overall survival and survival free of reoperation, acute myocardial infarction, thromboembolism, stroke or endocarditis.
Methods
- The BEST-VALVE independent clinical trial enrolled patients who underwent aortic valve replacement surgery between 2014 and 2017.
- Patients were randomly assigned to receive either a PERIMOUNT Magna Ease, Crown PRT or Trifecta valve (1:1:1).
- Haemodynamic performance was assessed through echocardiographic examination.
Results
Study population
- The study included 154 patients (PERIMOUNT Magna Ease valve: n=48; Crown PRT valve: n=51; Trifecta valve: n=55).with a mean age of 76.5 (inter-quartile range [IQR] 71.5–79.5) years, 59.7% of patients were male and the average EuroSCORE II was 2.3 (IQR 1.4–4).
- Elective procedures made up 8% of procedures and 87.7% of patients had severe aortic stenosis.
Haemodynamic performance 5 years after surgery
- Patients who received PERIMOUNT Magna Ease valve had the best haemodynamic outcomes (Table 1).
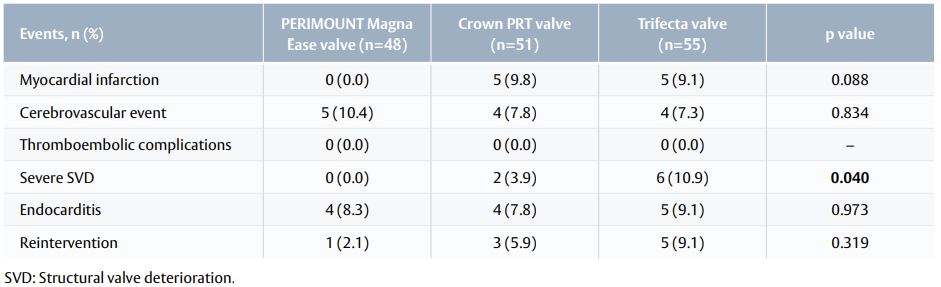
Serious adverse events
- Five years after surgery, serious adverse event rates were similar for all three study devices.
- The only exception was structural valve deterioration (SVD), which occurred significantly more often among patients with Trifecta valve (10.9%) compared with Crown PRT (3.9%) and Magna Ease (0%, p=0.04). (Table 2).
Surgical
- Overall and event-free survival were similar across all three study groups.
Table 1. Echocardiography outcomes 5 years after surgery.
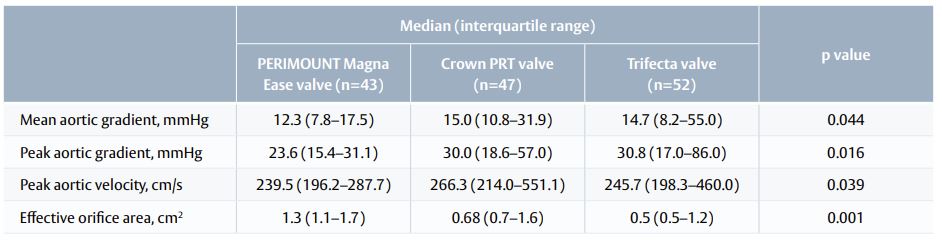
Table 2. Serious adverse events at 5 years after surgery.
Conclusion
After 5 years’ follow-up, the PERIMOUNT Magna Ease valve showed the best haemodynamic performance among the three study devices. Clinical outcomes were similar across cohorts.
Due to the small sample site and short duration of follow-up, the results of this study should be interpreted with caution.
This document is a summary of the Montero Cruces L et al. presentation, as presented at the EACTS congress, and covers key information including aim, type of study, methods, results, limitations and conclusions.
The full publication is available at:
Abbreviations:
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-5311 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.