December 6, 2022
 ~5m
~5mGeorges G, Bernard J, Pibarot P, Kalavrouziotis […]
Mid-term clinical and echocardiographic results of the INSPIRIS RESILIA aortic bioprosthesis – a retrospective comparison to the Carpentier-Edwards Magna Ease
Georges G, Bernard J, Pibarot P, Kalavrouziotis D and Mohammadi S.
Presented at 36th European Association for Cardio-Thoracic Surgery Annual Meeting, 2022.
Key points
- The INSPIRIS RESILIA valve showed comparable early and mid-term outcomes in patients who underwent surgical aortic valve replacement (SAVR), to the Carpentier-Edwards PERIMOUNT Magna Ease valve.
- The INSPIRIS RESILIA valve facilitated significantly lower mean pressure gradients at discharge, 1–3 months and 2 years compared with the Carpentier-Edwards PERIMOUNT Magna Ease valve.
Background information
- The number of younger patients receiving aortic bioprostheses is rising, creating a need for devices with better haemodynamic performance and durability.
- The INSPIRIS RESILIA valve is a relatively new device, for which only limited data are available currently.
Aims
- To evaluate the real-world mid-term clinical and echocardiographic outcomes in patients with INSPIRIS RESILIA valve.
Type of study
- A single-centre, retrospective registry study.
Endpoints
- Endpoints included haemodynamic outcomes, survival, and incidence of structural valve deterioration (SVD).
Methods
- The study included adults who underwent SAVR with an INSPIRIS RESILIA valve or a PERIMOUNT Magna Ease valve between January 2018 and July 2021 with ≥1 postoperative echocardiography examination.
- To account for baseline differences, patients were propensity score-matched for age, sex, prosthesis size, body surface area, body mass index and left ventricular ejection fraction.
Results
Patients
- The study originally enrolled 953 patients, 434 of whom were included in the propensity score-matched cohort (Table 1).
- The study achieved complete clinical follow-up (100%; median follow-up time: 2.5 years).
Table 1. Baseline characteristics of the propensity score-matched population.
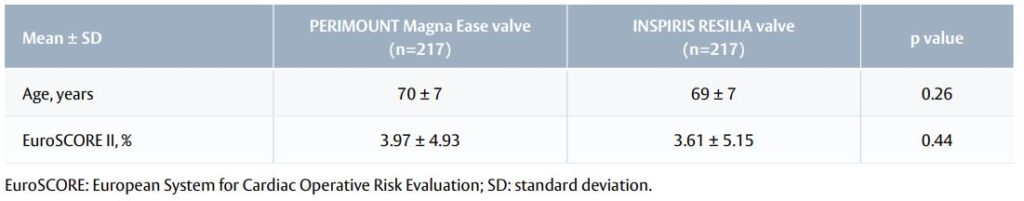
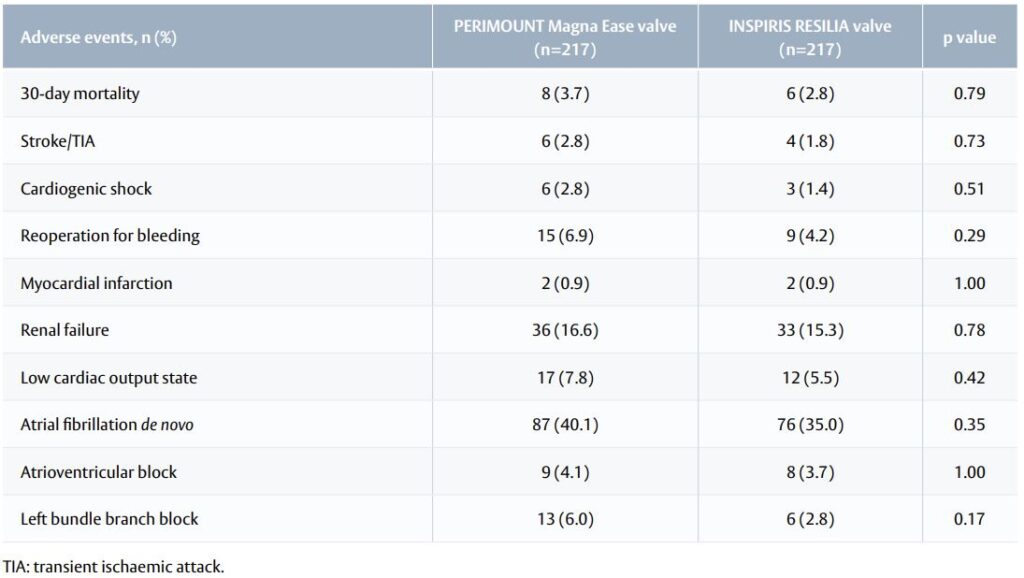
Table 2. Post-operative adverse events and complications for the propensity score-matched cohort
Clinical outcomes
- Early post-operative outcomes were similar between groups (Table 2).
- Survival was not significantly different between groups throughout follow-up (p=0.89).
- Readmission for cardiovascular events or stroke was more frequent among patients with PERIMOUNT Magna Ease valve (proportion of patients with readmission after 30 months: 6% with INSPIRIS RESILIA valve, 14% with PERIMOUNT Magna Ease valve; p=0.01).
- SVD was rare, with only two moderate SVD cases by Year 2 (both in the 21-mm INSPIRIS RESILIA valve group; p=0.87).
Haemodynamic outcomes
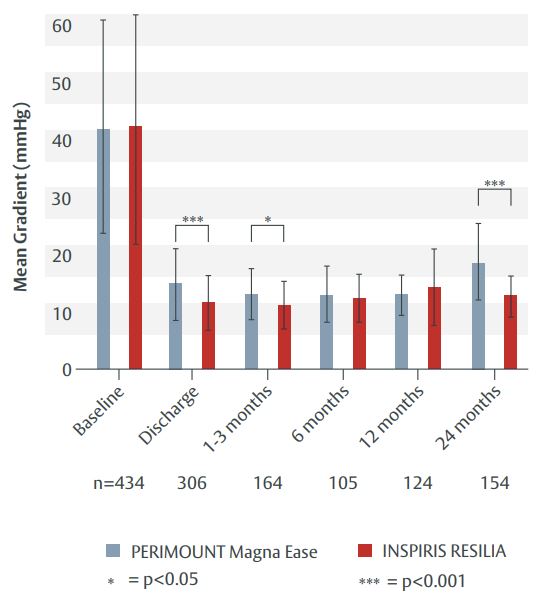
- The INSPIRIS RESILIA valve showed significantly lower mean pressure gradients at discharge, at 1–3 months and 2 years versus the PERIMOUNT Magna Ease valve (Figure 1).
Figure 1. Mean pressure gradient over time (propensity score-matched cohort).
Conclusion
The post-operative and mid-term outcomes were comparable in both study groups. The INSPIRIS RESILIA aortic valve showed stable mid-term haemodynamic performance, with a mean pressure gradient at 2 years that was significantly lower than with the PERIMOUNT Magna Ease valve. Long-term follow-up is underway.
This document is a summary of the Georges G et al. presentation as presented at the EACTS congress, and covers key information including aim, type of study, methods, results and conclusions.
The full publication is available at:
Abbreviations:

No clinical data are available that evaluate the long term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI, PERIMOUNT, PERIMOUNT Magna and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-5307 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere