December 6, 2022
 ~5m
~5mD’Onofrio A, Cibin G, Lorenzoni G, Tessari […]
Multicentre, large scale, propensity-weighted comparison of three aortic bioprostheses: conventional stented, new-generation stented and rapid deployment
D’Onofrio A, Cibin G, Lorenzoni G, Tessari C, Luzi G, Manzan E, Gerosa G and the INTU-ITA and RES-ITA Investigators.
Presented at 36th European Association for Cardio-Thoracic Surgery Annual Meeting, 2022.
Key points:
- All three valves demonstrated excellent haemodynamic outcomes with no differences in major postoperative complications.
- Operative times were lowest with the EDWARDS INTUITY valve; new pacemaker implantation was least common in patients receiving an INSPIRIS RESILIA valve.
- The EDWARDS INTUITY valve and the INSPIRIS RESILIA valve resulted in similar postoperative aortic gradients that were significantly lower than in patients with a Carpentier-Edwards PERIMOUNT Magna Ease valve.
Background information
- Several different aortic valve bioprostheses with different design characteristics and haemodynamic properties are available
- Options include conventional stented (CS), new-generation stented (NGS) and rapid deployment (RD) valves.
Aims
- To evaluate early clinical and haemodynamic outcomes of CS, NGS and RD aortic valve bioprostheses.
Type of study
- Multicentre, retrospective, observational study, with a propensity score weighting approach, that merged data from the INTU-ITA and the RES-ITA registry.
Endpoints
- Outcomes included operative times, postoperative complication rates and haemodynamic function.
Methods
- The study enrolled patients from 27 cardiac surgery centres who underwent surgical aortic valve replacement for aortic valve stenosis between January 2017 and December 2020 and received either a PERIMOUNT Magna Ease valve (CS), an INSPIRIS RESILIA valve (NGS) or an EDWARDS INTUITY (RD) valve.
Results
Study Population
- The study included 2,589 patients.
- Across the three groups, mean age was 72 (64–78) years, 40% patients were female and average body surface area was 1.83 (1.69–1.96) m2. Mean STS score was 1.66 (1.08–2.58) and 89% patients were in NYHA class >II.
- For the three-arm comparison, the EDWARDS INTUITY arm was chosen as the reference, with comparisons adjusted for factors including age, gender, comorbidities, STS score, NYHA and cardiac history.
- Most patients (65%, n=1,688) received an EDWARDS INTUITY valve; 605 (23%, n=605) received an INSPIRIS RESILIA valve and 296 (12%, n=296) a PERIMOUNT Magna Ease valve.
- INSPIRIS RESILIA was implanted in younger patients (Mean age: INSPIRIS RESILIA 60 [53–65] years; PERIMOUNT Magna Ease 72 [66–76] years; EDWARDS INTUITY 75 [66–76] years) with lower risk scores (STS score (%): INSPIRIS RESILIA 0.95 [0.65–1.52]; PERIMOUNT Magna Ease 1.3 [0.95–2.08]; EDWARDS INTUITY 1.9 [1.32–2.82])
Operative times
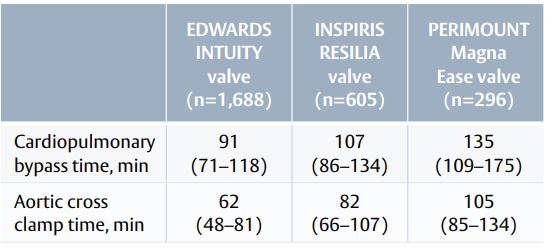
- The EDWARDS INTUITY valve allowed for shorter procedural times than the other bioprostheses (Table 1).
Table 1. Median operative times
Postoperative complications
- All three study groups had a device success rate of 96%.
- The incidence of major postoperative complications was similar across all study groups.
- New pacemaker implantations were significantly less frequent in the INSPIRIS RESILIA valve group (rate of 2%) than in the other study arms (6% in both the EDWARDS INTUITY valve and PERIMOUNT Magna Ease valve group).
Haemodynamic function
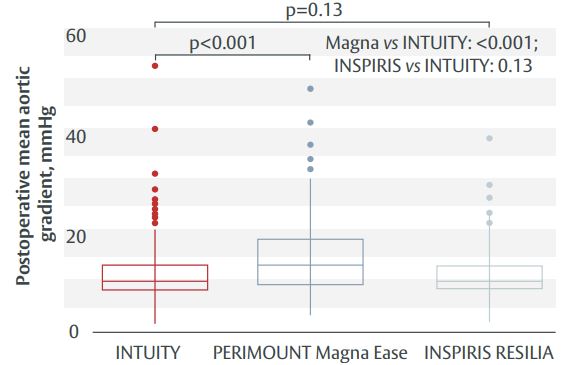
- Haemodynamic outcomes were better with the EDWARDS INTUITY valve compared with the PERIMOUNT Magna Ease valve, whereas there was no difference between the INSPIRIS RESILIA valve and EDWARDS INTUITY (Figure 1).
Figure 1. Postoperative mean aortic gradient.
Conclusion
All three bioprostheses gave excellent haemodynamic and clinical outcomes, with significantly lower gradients with EDWARDS INTUITY valve vs PERIMOUNT Magna Ease valve. There was no difference in gradients between EDWARDS INTUITY and INSPIRIS RESILIA valves. Operative times were shortest with the EDWARDS INTUITY valve and the incidence of pacemaker implantation lowest among patients with INSPIRIS RESILIA valve. The rate of major postoperative complications was similar across all three study groups.
This document is a summary of the D’Onofrio A et al. presentation and covers key information including aim, type of study, methods, results, limitations and conclusions.
The full publication is available at:
Abbreviations:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Important safety information:
Use of the EDWARDS INTUITY Elite valve system may be associated with new or worsened conduction disturbances, which may require a permanent cardiac pacemaker implant (PPI). The rate of PPI for the EDWARDS INTUITY Elite valve is within the range reported in the literature for various rapid deployment valves, but higher than that reported for surgical aortic valves. Physicians should assess the benefits and risks of the EDWARDS INTUITY Elite valve prior to implantation. See instructions for use for additional information.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, EDWARDS INTUITY, EDWARDS INTUITY Elite, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI,
PERIMOUNT, PERIMOUNT Magna and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2022 Edwards Lifesciences Corporation. All rights reserved. PP–EU-5309 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.