What is RESILIA tissue and how did it get here?
Angela De La Fuente
EACTS annual meeting, 2023






November 20, 2023
Angela De La Fuente
EACTS annual meeting, 2023
Presenter: Angela De La Fuente
Prof. Onorati
2023 EACTS annual meeting
Presenter: Prof. Onorati
Dr. Joseph Bavaria
2023 EACTS annual meeting
Presenter: Dr. Bavaria
March 17, 2022
Insights from INDURE registry
Prof. Ruggero De Paulis
Presenter: Prof. De Paulis
November 2, 2023
Meuris B, Roussel JC, Borger MA et al.
Interdisciplinary CardioVascular and Thoracic Surgery. 2023; doi:10.1093/icvts.ivad115.
–Haemodynamic performance assessment.
– Durability evaluation of INSPIRIS RESILIA aortic valve (composite endpoint of time-related valve safety [VARC-2; adjudicated by an independent clinical events committee] and structural valve deterioration [SVD] stage 3 [VARC-3; based on a standardised Core Lab adjudicated assessment]).
– Clinical outcomes: all cause, cardiovascular and valve-related mortality; valve-related dysfunction; need for repeat procedures; permanent pacemaker implantation; acute kidney injury stage 2 or 3; and New York Heart Association (NYHA) functional class.
– Evaluation of QoL using Kansas City Cardiomyopathy Questionnaire (KCCQ) and Short Form-12 Health Survey (SF-12v2).
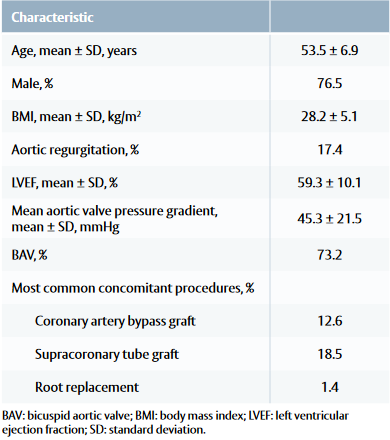
Baseline and procedural characteristic
• Common comorbidities included arterial hypertension, coronary artery disease and type II diabetes.
Table 1: Patient baseline and procedural characteristics
Early (30 days) and 1-year outcomes
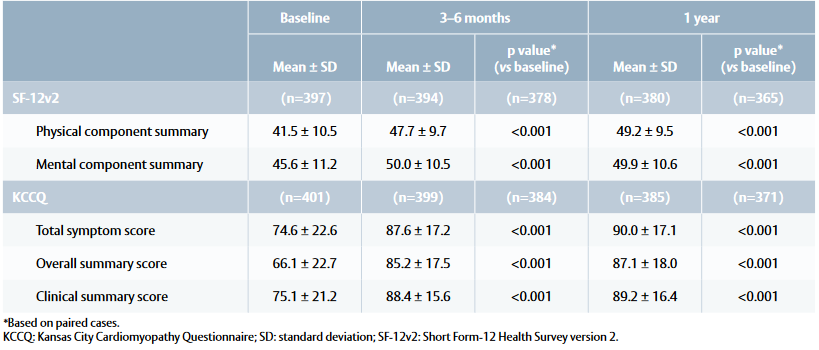
Quality-of-life outcomes
Table 2: Quality of life outcomes at baseline, 3–6 months and 1 year
Bartus K, Litwinowicz R, Bilewska A et al. Intermediate term outcomes after aortic valve replacement with a novel RESILIA™ tissue bioprosthesis. J Thorac Dis. 2019; 11: 3039–46.
Useini D, Schlomicher M, Haldenwang P et al. Early results after aortic valve replacement using last generation bioprosthetic aortic valve. Heart Surg Forum. 2021; 24: E598–962.
Bavaria JE, Griffith B, Heimansohn DA et al. Five year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg. 2022; 115: 1429–36.
Fukunaga N, Yoshida S, Shimoji A et al. Hemodynamic performance of INSPIRIS RESILIA aortic bioprosthesis for severe aortic stenosis: 2 year follow-up in Japanese cohort. J Artif Organs. 2022; 25: 323–8.
This document is a summary of the Meuris B et al. paper
and covers key information including aim, type of study,
methods, results and conclusion.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential
adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Refer to device instructions for use for important warnings related to VFit technology. These features have not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in valve procedures. VFit technology is available on sizes 19–25 mm.
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, NSPIRIS, RESILIA, RESILIA and VFit are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6745 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Meuris B, Roussel JC, Borger MA et al.
Interdisciplinary CardioVascular and Thoracic Surgery. 2023; doi:10.1093/icvts.ivad115.
–Haemodynamic performance assessment.
– Durability evaluation of INSPIRIS RESILIA aortic valve (composite endpoint of time-related valve safety [VARC-2; adjudicated by an independent clinical events committee] and structural valve deterioration [SVD] stage 3 [VARC-3; based on a standardised Core Lab adjudicated assessment]).
– Clinical outcomes: all cause, cardiovascular and valve-related mortality; valve-related dysfunction; need for repeat procedures; permanent pacemaker implantation; acute kidney injury stage 2 or 3; and New York Heart Association (NYHA) functional class.
– Evaluation of QoL using Kansas City Cardiomyopathy Questionnaire (KCCQ) and Short Form-12 Health Survey (SF-12v2).
Baseline and procedural characteristic
• Common comorbidities included arterial hypertension, coronary artery disease and type II diabetes.
Table 1: Patient baseline and procedural characteristics

Early (30 days) and 1-year outcomes
Quality-of-life outcomes
Table 2: Quality of life outcomes at baseline, 3–6 months and 1 year

Bartus K, Litwinowicz R, Bilewska A et al. Intermediate term outcomes after aortic valve replacement with a novel RESILIA™ tissue bioprosthesis. J Thorac Dis. 2019; 11: 3039–46.
Useini D, Schlomicher M, Haldenwang P et al. Early results after aortic valve replacement using last generation bioprosthetic aortic valve. Heart Surg Forum. 2021; 24: E598–962.
Bavaria JE, Griffith B, Heimansohn DA et al. Five year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg. 2022; 115: 1429–36.
Fukunaga N, Yoshida S, Shimoji A et al. Hemodynamic performance of INSPIRIS RESILIA aortic bioprosthesis for severe aortic stenosis: 2 year follow-up in Japanese cohort. J Artif Organs. 2022; 25: 323–8.
This document is a summary of the Meuris B et al. paper
and covers key information including aim, type of study,
methods, results and conclusion.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential
adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Refer to device instructions for use for important warnings related to VFit technology. These features have not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in valve procedures. VFit technology is available on sizes 19–25 mm.
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, NSPIRIS, RESILIA, RESILIA and VFit are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6745 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Authors: Meuris B, Roussel JC, Borger MA et al.
Francica A, Tonelli F, Rossetti C, Galeone A, Perrone F, Luciani GB and Onorati F.
Journal of Clinical Medicine. 2023; 12: 2077.
Baseline characteristics
Postoperative outcomes
– Permanent pacemaker implantation: 3.3% PERIMOUNT Magna Ease valve versus 1.6% INSPIRIS RESILIA valve (p=0.4).
– Type 1 stroke: 0.8% in both groups.
– Paroxysmal atrial fibrillation was the most common complication (22.1% PERIMOUNT Magna Ease valve vs 24.6% INSPIRIS RESILIA valve, p=0.88).
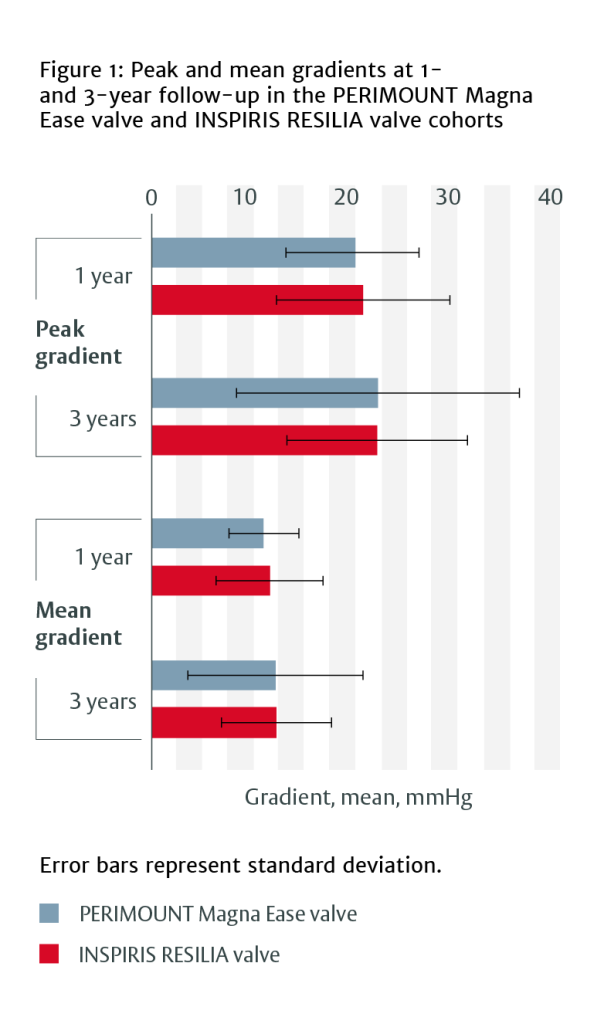
Haemodynamic performance
– Between January 2010 and December 2012, 238 patients underwent AVR with a PERIMOUNT Magna Ease valve.
– Between September 2017 and January 2022, 192 patients underwent AVR with an INSPIRIS RESILIA valve.
A propensity score-matched analysis was performed to account for baseline differences, resulting in 122 matched pairs.
Echocardiographic data was collected 1 and 3 years post surgery.
At 1 year, patients with INSPIRIS RESILIA valves had lower intraventricular septum thickness measurements (12.1 ± 2.0 mm vs 13.2 ± 2.1 mm, p=0.011) and systolic pulmonary artery pressure (26.8 ± 7.6 mmHg vs 34.3 ± 9.3 mmHg, p=0.001) than those with PERIMOUNT Magna Ease valves.
Severe structural valve deterioration occurred in four patients (all <50 years old) with PERIMOUNT Magna Ease valve and no patients with INSPIRIS RESILIA valve.
When stratified by valve size, there were no differences in haemodynamic behaviour between the two valves for any valve size category.
This document is a summary of the Francica A et al. paper and covers key information including aim, type of study, methods, results and conclusion.
The full pubblication is available at:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6466 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Francica A, Tonelli F, Rossetti C, Galeone A, Perrone F, Luciani GB and Onorati F.
Journal of Clinical Medicine. 2023; 12: 2077.
Baseline characteristics
Postoperative outcomes
– Permanent pacemaker implantation: 3.3% PERIMOUNT Magna Ease valve versus 1.6% INSPIRIS RESILIA valve (p=0.4).
– Type 1 stroke: 0.8% in both groups.
– Paroxysmal atrial fibrillation was the most common complication (22.1% PERIMOUNT Magna Ease valve vs 24.6% INSPIRIS RESILIA valve, p=0.88).
Haemodynamic performance
– Between January 2010 and December 2012, 238 patients underwent AVR with a PERIMOUNT Magna Ease valve.
– Between September 2017 and January 2022, 192 patients underwent AVR with an INSPIRIS RESILIA valve.
A propensity score-matched analysis was performed to account for baseline differences, resulting in 122 matched pairs.
Echocardiographic data was collected 1 and 3 years post surgery.
At 1 year, patients with INSPIRIS RESILIA valves had lower intraventricular septum thickness measurements (12.1 ± 2.0 mm vs 13.2 ± 2.1 mm, p=0.011) and systolic pulmonary artery pressure (26.8 ± 7.6 mmHg vs 34.3 ± 9.3 mmHg, p=0.001) than those with PERIMOUNT Magna Ease valves.
Severe structural valve deterioration occurred in four patients (all <50 years old) with PERIMOUNT Magna Ease valve and no patients with INSPIRIS RESILIA valve.
When stratified by valve size, there were no differences in haemodynamic behaviour between the two valves for any valve size category.

This document is a summary of the Francica A et al. paper and covers key information including aim, type of study, methods, results and conclusion.
The full pubblication is available at:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6466 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Authors: Francica A, Tonelli F, Rossetti C, Galeone A, Perrone F, Luciani GB and Onorati F.
October 31, 2022
Prof. Kalavrouziotis
Edwards Lifesciences at EACTS 2022: State of the art in the treatment of aortic valve regurgitation in young patients
Presenter: Dr. Kalavrouziotis
June 1, 2022
Prof. Augusto d’Onofrio
MVT Aortic 2022, vol.1
– Valve-in-valve, TAVI in TAVI, SAVR after TAVI… familiarize yourself with the mid-to-long-term data for each valve type combination, and transparently inform the patients of not only the benefits but also the risks
– Even the experts say, extracting an infected TAVI is extremely difficult, so be sure to choose the original valve wisely
Presenter: Prof. D’Onofrio
December 15, 2023
Tom Nguyen
Presenter: Dr. Nguyen
May 29, 2022
Prof. Bart Meuris
MVT Aortic 2022, vol.1
Presenter: Prof. Meuris
September 16, 2022
Live Case filmed at Hezrklinik Hirslanden, with:
Presenter: Prof. Grunenfelder
July 7, 2023
Prof. Treede
Aortic Highlights course, 2023
Presenter: Prof. Treede
October 31, 2022
Prof. Anselmi
Edwards Lifesciences at EACTS 2022: Aortic valve disease: Patient centric management in elderly population
Presenter: Prof. Anselmi
November 30, 2021
Prof. Kocher – EACTS 2021
Presenter: Prof. D’Onofrio
November 2, 2023
Porto A, Stolpe G, Badaoui R et al.
Frontiers in Cardiovascular Medicine. 2023; doi:10.3389/fcvm.2023.1196447.
Baseline and procedural characteristics
Clinical outcomes at 1 year
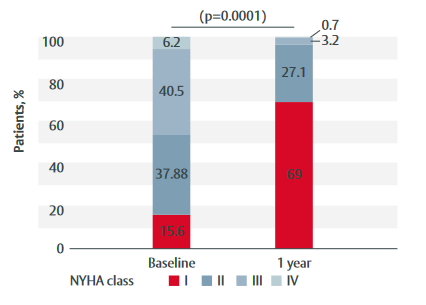
Figure 1: Change in NYHA class from baseline to 1-year follow-up in patients treated with the INSPIRIS RESILIA valve
Haemodynamic properties
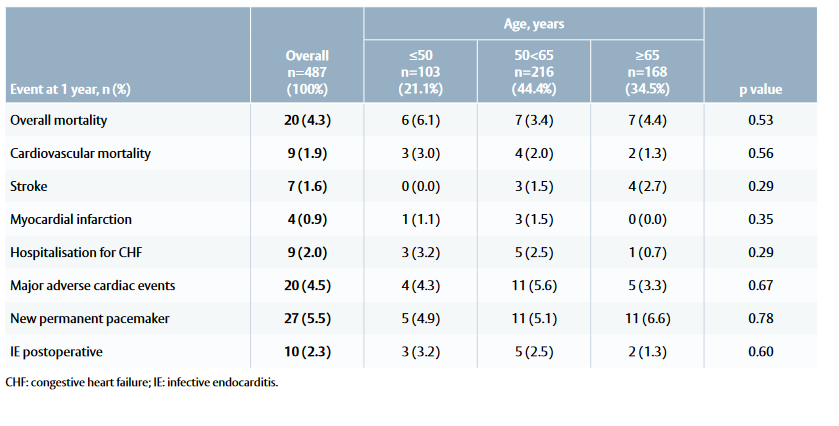
Table 1: Mortality and morbidity outcomes at 1 year among patients treated with the INSPIRIS RESILIA valve
Infective endocarditis
This document is a summary of the Porto A et al. paper
and covers key information including aim, type of study,
methods, results and conclusions
The abstract is available at:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, EDWARDS INTUITY, EDWARDS INTUITY Elite, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI,
PERIMOUNT, PERIMOUNT Magna and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6744 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Porto A, Stolpe G, Badaoui R et al.
Frontiers in Cardiovascular Medicine. 2023; doi:10.3389/fcvm.2023.1196447.
Baseline and procedural characteristics
Clinical outcomes at 1 year
Figure 1: Change in NYHA class from baseline to 1-year follow-up in patients treated with the INSPIRIS RESILIA valve

Haemodynamic properties
Table 1: Mortality and morbidity outcomes at 1 year among patients treated with the INSPIRIS RESILIA valve

Infective endocarditis
This document is a summary of the Porto A et al. paper
and covers key information including aim, type of study,
methods, results and conclusions
The abstract is available at:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, EDWARDS INTUITY, EDWARDS INTUITY Elite, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI,
PERIMOUNT, PERIMOUNT Magna and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6744 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Authors: Porto A, Stolpe G, Badaoui R et al.
El-Sayed Ahmad A, Giammarino S, Salamate S, Fehske W, Sirat S, Amer M, Bramlage P, Bakhtiary F and Doss M.
Journal of Cardiac Surgery. 2022; 37: 4833–40
Study population
Mortality and valve-related complications
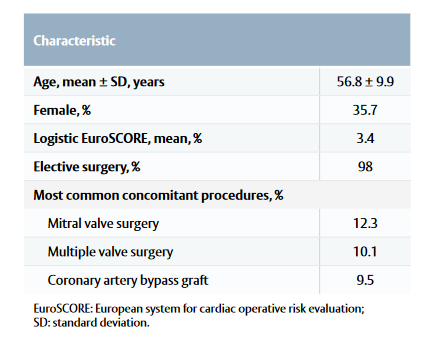
Table 1: Patient baseline characteristics
Haemodynamic properties
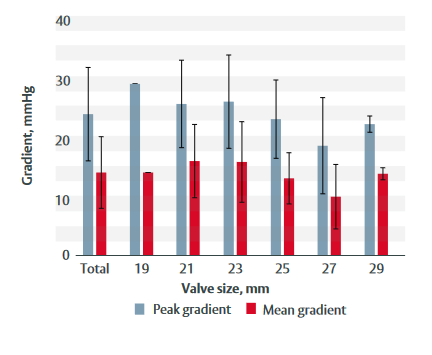
Figure 1: Peak and mean gradients at 3-year follow-up for each valve size
This document is a summary of the El-Sayed Ahmed A et al. paper and covers key information including aim, type of study, methods, results and conclusions.
The full abstract is available at:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6062 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
El-Sayed Ahmad A, Giammarino S, Salamate S, Fehske W, Sirat S, Amer M, Bramlage P, Bakhtiary F and Doss M.
Journal of Cardiac Surgery. 2022; 37: 4833–40
Study population
Mortality and valve-related complications
Table 1: Patient baseline characteristics

Haemodynamic properties
Figure 1: Peak and mean gradients at 3-year follow-up for each valve size

This document is a summary of the El-Sayed Ahmed A et al. paper and covers key information including aim, type of study, methods, results and conclusions.
The full abstract is available at:

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6062 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Authors: El-Sayed Ahmad A, Giammarino S, Salamate S, Fehske W, Sirat S, Amer M, Bramlage P, Bakhtiary F and Doss M.
Clinical Trials and Studies ()
![]() 08:+8 m
08:+8 m

Mid-term outcomes of INSPIRIS RESILIA valve compared to Magna Ease valve
Presenter: Prof. Onorati
![]() 12:58 m
12:58 m

7-year COMMENCE trial data
Presenter: Dr. Bavaria
![]() 05:02 m
05:02 m

Durability of bioprosthetic valves in patients under the age of 60 years - focus on the INDURE registry
Presenter: Prof. De Paulis
![]() 10 Min
10 Min

1-year follow-up from the prospective INDURE registry - Durability of bioprosthetic aortic valve replacement in patients under the age of 60 years
Authors: Meuris B, Roussel JC, Borger MA et al.
Biological valve in Young Patient Population ()
![]() 10 Min
10 Min

PERIMOUNT Magna Ease vs INSPIRIS RESILIA valve: A PS-matched analysis of the hemodynamic performances in patients below 70 years of age
Authors: Francica A, Tonelli F, Rossetti C, Galeone A, Perrone F, Luciani GB and Onorati F.
![]() 11:18 m
11:18 m

Assessment of a new bioprosthetic valve to treat young patients with bicuspid aortic valve
Presenter: Dr. Kalavrouziotis
Single-center experiences ()
![]() 12:24 m
12:24 m

Not All Biological Valves Are Equal: A Single-Center Experience
Presenter: Prof. Anselmi
![]() 13:11 m
13:11 m

Single center experience with INSPIRIS RESILIA aortic valve
Presenter: Prof. D’Onofrio
![]() 10 Min
10 Min

1-year clinical outcomes following INSPIRIS RESILIA aortic valve implantation in 487 young patients with severe aortic stenosis
Authors: Porto A, Stolpe G, Badaoui R et al.
![]() 10 Min
10 Min

Clinical performance of a novel bioprosthetic surgical aortic valve in a German high-volume center
Authors: El-Sayed Ahmad A, Giammarino S, Salamate S, Fehske W, Sirat S, Amer M, Bramlage P, Bakhtiary F and Doss M.
About
Overview
 ~08:51m
~08:51m ~36:08m
~36:08m ~21:18m
~21:18m ~19:05m
~19:05m ~17:43m
~17:43m ~32:07m
~32:07m ~45:35m
~45:35m