November 2, 2023
 ~5m
~5mFrancica A, Tonelli F, Rossetti C, Galeone […]
PERIMOUNT Magna Ease vs INSPIRIS RESILIA valve: A PS-matched analysis of the hemodynamic performances in patients below 70 years of age
Francica A, Tonelli F, Rossetti C, Galeone A, Perrone F, Luciani GB and Onorati F.
Journal of Clinical Medicine. 2023; 12: 2077.
Key points
- This is the first propensity-matched analysis comparing the haemodynamic performance of the INSPIRIS RESILIA valve with the Carpentier-Edwards PERIMOUNT Magna Ease valve at mid-term follow-up in patients under 70 years of age.
- For up to 3 years post surgery, the haemodynamic performance was comparable between the two valve types in this patient population; however, the INSPIRIS RESILIA valve was associated with lower intraventricular septum thickness measurements and systolic pulmonary artery pressure.
Background information
- The PERIMOUNT Magna Ease valve has been implanted worldwide during the last 10 years and has achieved excellent long- erm clinical and haemodynamic outcomes.
- The INSPIRIS RESILIA valve was introduced with the aim of improving valve durability by reducing calcification; 1 but how the haemodynamics of this valve compare with the PERIMOUNT Magna Ease valve has not previously been evaluated.
Aim
- To compare the haemodynamic performance of the PERIMOUNT Magna Ease valve with the INSPIRIS RESILIA valve in patients under 70 years.
Type of study
- A retrospective single-centre study with propensity-matched analysis.
Endpoints
- The primary endpoint was to compare the short- and mid-term haemodynamic performances of the PERIMOUNT Magna Ease valve and the INSPIRIS RESILIA valve.
- Secondary endpoints included:
– Haemodynamic performance stratified by prosthetic valve size.
– Postoperative outcomes.
Results
Baseline characteristics
- The two propensity score-matched populations were similar in age (mean 57.4 ± 10.1 years) and risk profile (EuroSCORE II 2.7 ± 2.4%).
- The most common comorbidity was hypertension (54.5%), and 31.6% of patients were in New York Heart Association functional class III or IV.
Postoperative outcomes
- Postoperative outcomes were comparable between the two populations.
- The overall incidence of major complications was low:
– No procedural or periprocedural mortality.– Permanent pacemaker implantation: 3.3% PERIMOUNT Magna Ease valve versus 1.6% INSPIRIS RESILIA valve (p=0.4).
– Type 1 stroke: 0.8% in both groups.
– Paroxysmal atrial fibrillation was the most common complication (22.1% PERIMOUNT Magna Ease valve vs 24.6% INSPIRIS RESILIA valve, p=0.88).
Haemodynamic performance
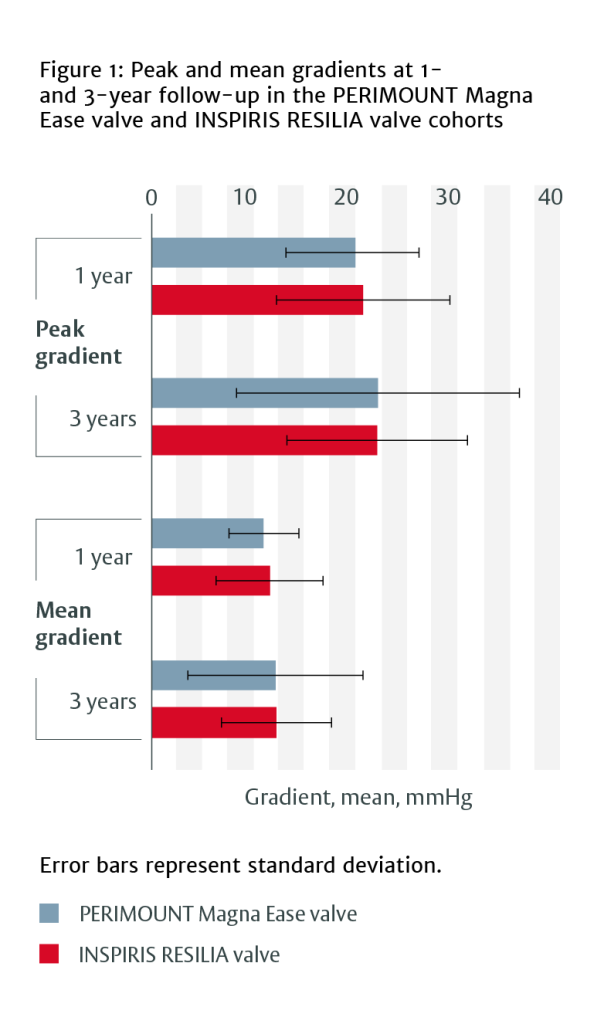
- PERIMOUNT Magna Ease valve and INSPIRIS RESILIA valve showed similar haemodynamic performance at 1-year and 3-year follow-up in terms of peak and mean gradient (Figure 1) and left ventricular volumes and diameters.
Methods
- The study population consisted of patients who were aged between 18 and 70 years and underwent aortic valve replacement (AVR) at the Division of Cardiac Surgery, University of Verona.
– Between January 2010 and December 2012, 238 patients underwent AVR with a PERIMOUNT Magna Ease valve.
– Between September 2017 and January 2022, 192 patients underwent AVR with an INSPIRIS RESILIA valve. A propensity score-matched analysis was performed to account for baseline differences, resulting in 122 matched pairs.
Echocardiographic data was collected 1 and 3 years post surgery.
At 1 year, patients with INSPIRIS RESILIA valves had lower intraventricular septum thickness measurements (12.1 ± 2.0 mm vs 13.2 ± 2.1 mm, p=0.011) and systolic pulmonary artery pressure (26.8 ± 7.6 mmHg vs 34.3 ± 9.3 mmHg, p=0.001) than those with PERIMOUNT Magna Ease valves.
Severe structural valve deterioration occurred in four patients (all <50 years old) with PERIMOUNT Magna Ease valve and no patients with INSPIRIS RESILIA valve.
When stratified by valve size, there were no differences in haemodynamic behaviour between the two valves for any valve size category.
Limitations
- This was a retrospective study.
- The two patient groups were treated almost 10 years apart, and patients with INSPIRIS RESILIA valves had a shorter follow-up.
- There was no standardised protocol for echocardiography, although all labs complied with guidelines.
- Data retrieval was challenging due to the study taking place during the COVID-19 pandemic.
Conclusion
- The INSPIRIS RESILIA valve achieved the same efficacy and safety outcomes as the PERIMOUNT Magna Ease valve at mid-term follow-up in this propensity-matched analysis of patients under 70 years of age.
Reference
- Flameng W, Hermans H, Verbeken E et al.
A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015; 149: 340–5.
This document is a summary of the Francica A et al. paper and covers key information including aim, type of study, methods, results and conclusion.
The full pubblication is available at:
Abbreviations

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6466 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.