November 2, 2023
 ~5m
~5mPorto A, Stolpe G, Badaoui R et […]
One-year clinical outcomes following Edwards INSPIRIS RESILIA aortic valve implantation in 487 young patients with severe aortic stenosis: A single-centre experience
Porto A, Stolpe G, Badaoui R et al.
Frontiers in Cardiovascular Medicine. 2023; doi:10.3389/fcvm.2023.1196447.
Key points:
- The management of aortic stenosis in young patients is an important topic.
- The INSPIRIS RESILIA valve demonstrated encouraging survival, clinical and haemodynamic outcomes at 1-year follow-up in a large, single-center prospective study of consecutive young patients with severe aortic stenosis (AS).
Background information
- Aortic bioprostheses are increasingly being used in younger patients with severe AS, despite the risk of accelerated structural valve deterioration (SVD) and subsequent need for reintervention, especially in patients under 65 years of age.
- The INSPIRIS RESILIA valve, a bovine pericardial tissue valve with a special integrity preservation technology, represents a promising solution to SVD.
- Early clinical experience with the INSPIRIS RESILIA valve has shown encouraging results in terms of early safety and efficacy of aortic valve replacement (AVR).
- Intermediate-term outcomes have shown the absence of early thrombosis events and non-calcific valve deterioration.1-2
Aim
- To evaluate the 1-year outcomes of the INSPIRIS RESILIA aortic valve in a population of young patients who underwent AVR for severe AS.
Type of study
- A prospective single-centre study.
Results
Baseline and procedural characteristics
- Mean (± standard deviation [SD]) age was 58.2 ± 11.5 years and 75.2% were male.
- Most of the interventions were elective (endocarditis 18.7%; 14.5% redo surgery; and 31.2% AS without aortic regurgitation), with a mean (± SD) EuroSCORE II of 4.8 ± 7.9%.
- Patients 50 years of age or younger had a higher EuroSCORE II (5.3 ± 9.6%) and were more likely to present with preoperative IE (29.2% vs 18.5% for the 50<65 group and 12.5% for the ≥65 group).
- 210 patients (43.1%) had a concomitant procedure performed perioperatively:
– 23.4% had aortic replacement.
– 9.2% had mitral valve surgery replacement or repair.
– 7.4% had coronary artery bypass graft.
Clinical outcomes at 1 year
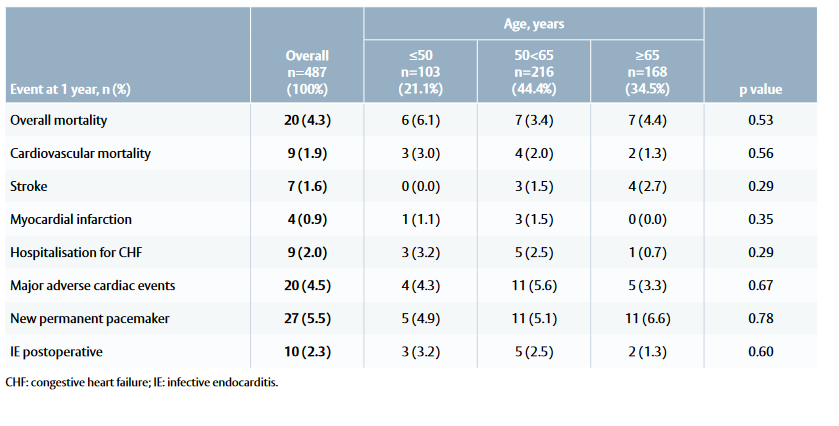
- The Kaplan–Meier estimated survival rate was 96.4%.
- There was no significant difference in either overall or cardiac mortality, or major cardiac events between the three age groups (Table 1).
Endpoints
- The primary endpoint was overall mortality at 1 year.
- Secondary endpoints included:
– Cardiovascular mortality.
– Major adverse cardiac events (defined by rehospitalisation for congestive heart failure, stroke or myocardial infarction).
– Evolution of New York Heart Association (NYHA) functional status.
– Haemodynamic follow- up.
– Mortality and morbidity of infective endocarditis (IE).
- All outcomes were defined according to the Valve Academic Research Consortium-2 consensus document.
Methods
- A total of 487 consecutive patients with severe AS who received the INSPIRIS RESILIA valve between June 2017 and July 2021 at La Timone Hospital in Marseille, France, were included in the study.
- Clinical assessment and transthoracic echocardiography were performed preoperatively and at 1 year postoperatively.
- Subgroup analyses were conducted based on age (≤50 years, 50<65 years and ≥65 years) and preoperative IE status.
Figure 1: Change in NYHA class from baseline to 1-year follow-up in patients treated with the INSPIRIS RESILIA valve
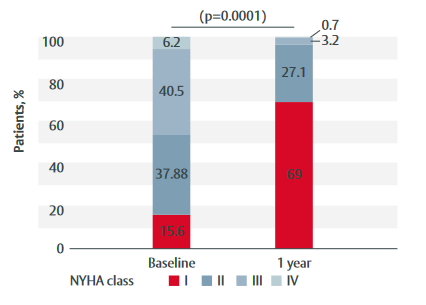
- Functional status was significantly improved (p<0.0001) (Figure 1).
- Severe patient–prosthesis mismatch and severe intravalvular regurgitation occurred in 1.2% and 0.6% of patients, respectively.
Haemodynamic properties
- Haemodynamic performance was significantly improved (baseline vs 1-year postoperative data; median [interquartile range]; p<0.001 for all):
– Aortic valve area index: 0.4 (0.4, 0.5) versus 1.0 (0.8, 1.3).
– Mean gradient: 49 mmHg (43, 55) versus 9 mmHg (7, 12).
– Peak velocity: 4.4 m/s (4.0, 4.9) versus 2.1 m/s (1.8, 2.4). - There were no significant differences in these parameters between the age groups.
Table 1: Mortality and morbidity outcomes at 1 year among patients treated with the INSPIRIS RESILIA valve
Infective endocarditis
- The Kaplan–Meier estimated survival rates at 1 year were 97.9% for the IE-free group and 89.5% for the IE group.
- Excluding IE-related causes, no SVD and valve failure requiring redo surgery were reported.
Limitations
- This was a single-arm study without comparative groups.
- Patients were only followed up for 1 year; therefore, long-term durability cannot be fully assessed.
- There was a high prevalence of preoperative IE in patients 50 years and younger, which explains the high rate of adverse events in this cohort.
– While the data seem to favour the INSPIRIS RESILIA valve in this population, the number of patients is too low to draw any conclusions.
Conclusions
- The INSPIRIS RESILIA valve provides encouraging clinical outcomes with excellent 1-year survival rates and good haemodynamic performance.
- Further studies are needed to assess long-term valve durability.
References
- Bartuś K, Litwinowicz R, Kuśmierczyk M et al. Primary safety and effectiveness feasibility study after surgical aortic valve replacement with a new generation bioprosthesis: One-year outcomes.
Kardiol Pol. 2018; 76: 618–24. - Johnston DR, Griffith BP, Puskas JD et al. Intermediate-term outcomes of aortic valve replacement using a bioprosthesis with a novel tissue. J Thorac Cardiovasc Surg. 2021; 162: 1478–85.
This document is a summary of the Porto A et al. paper
and covers key information including aim, type of study,
methods, results and conclusions
The abstract is available at:
Abbreviations

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, EDWARDS INTUITY, EDWARDS INTUITY Elite, INSPIRIS, INSPIRIS RESILIA, Magna, Magna Ease, PERI,
PERIMOUNT, PERIMOUNT Magna and RESILIA are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6744 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.