November 2, 2023
 ~5m
~5mEl-Sayed Ahmad A, Giammarino S, Salamate S, […]
Clinical performance of a novel bioprosthetic surgical aortic valve in a German high-volume center
El-Sayed Ahmad A, Giammarino S, Salamate S, Fehske W, Sirat S, Amer M, Bramlage P, Bakhtiary F and Doss M.
Journal of Cardiac Surgery. 2022; 37: 4833–40
Key points:
- The mid-term follow-up results of this single- centre study, in a patient population with a mean age of 56.8 years, indicate favourable procedural outcomes, with adequate durability and no cases of structural valve deterioration (SVD).
- At Year 3, haemodynamic performance was stable and adequate.
- INSPIRIS RESILIA aortic valve may represent a bioprosthesis suitable for younger patients, giving excellent results.
Background information
- Surgical aortic valve replacement (SAVR) using bioprosthetic valves is becoming increasingly popular in younger patients.
- The INSPIRIS RESILIA aortic valve features a tri-leaflet bovine pericardial tissue mounted under a flexible frame and has been developed to reduce calcification, therefore, increasing durability.
Aim
- To analyse mid-term follow-up data in patients who underwent SAVR with an INSPIRIS RESILIA bioprosthetic heart valve at the Heart Centre in Siegberg-Wuppertal.
Type of study
- A single-centre, non- randomised retrospective study.
Endpoints
- The primary endpoint was the haemodynamic properties of the RESILIA tissue valve at mid-term follow-up.
- Secondary objectives included mortality and valve-related complications (according to Valve Academic Research Consortium-3 criteria).1
Limitations
- This is a retrospective, non- randomised, single-centre study with a small sample size and no control group.
Methods
- All patients with aortic valve disease who received an INSPIRIS RESILIA aortic valve between April 2017 and December 2019 at the Division of Cardiac Surgery, Heart Centre Siegberg-Wuppertal, University Witten- Herdecke, Germany (n=154) were included.
- Follow-up data were collected in the clinic or by telephone during the 3-month closing interval ending in April 2021.
- Echocardiographic data were collected twice a year.
Results
Study population
- Mean (± standard deviation) patient follow-up was 2.05 ± 0.77 years.
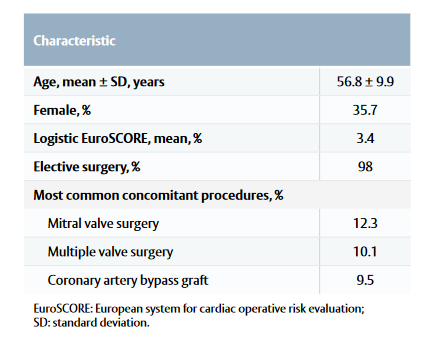
- Baseline characteristics are listed in Table 1.
Mortality and valve-related complications
- 30-day, 1-year and 3-year freedom from mortality were 98.7%, 97.3% and 87.7%, respectively; the rate of valve-related mortality was 0%.
- There were no cases of SVD or valve-related thrombosis and one (0.6%) case of reoperation due to endocarditis.
Table 1: Patient baseline characteristics
Haemodynamic properties
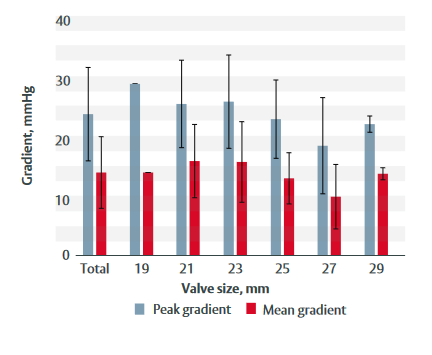
- At last follow-up:– Effective orifice area was 1.9 cm2. – Peak gradient was 23.6 mmHg. – Mean gradient was 13.9 mmHg.
- Figure 1 shows peak and mean gradients at 3-year follow-up by valve size.
Figure 1: Peak and mean gradients at 3-year follow-up for each valve size
Conclusions
- The INSPIRIS RESILIA valve shows adequate mid-term durability and haemodynamic performance.
- Further studies are required to assess long-term durability and efficacy, especially in younger patients.
Reference
- Genereux P, Piazza N, Alu MC et al. Valve Academic Research Consortium 3: Updated endpoint definitions for aortic valve clinical research.
Eur Heart J. 2021; 42: 1825–57
This document is a summary of the El-Sayed Ahmed A et al. paper and covers key information including aim, type of study, methods, results and conclusions.
The full abstract is available at:
Abbreviations

No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Edwards, Edwards Lifesciences, the stylized E logo, Carpentier-Edwards, Carpentier-Edwards PERIMOUNT, Carpentier-Edwards PERIMOUNT Magna Ease, Magna, Magna Ease, PERI, PERIMOUNT and PERIMOUNT Magna are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners.
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6062 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.