Decision making in primary MR: which patient for which therapy
Dr. Dahle
Mitral Highlights course, 2023
Dr. Farber – MVT Intermediate 2021
Mr. Steve Livesey
Be courageous, be experienced, be ethical … according to the Guidelines!
The highly anticipated 2021 European Society of Cardiology (ESC) and European Association for Cardio-Thoracic Surgery (EACTS) guidelines for the management of valvular heart disease have finally landed.
We are excited to bring you a summary of the main updates, plus the new materials coming soon to support you and your teams in understanding and implementing the changes.
Key points include:

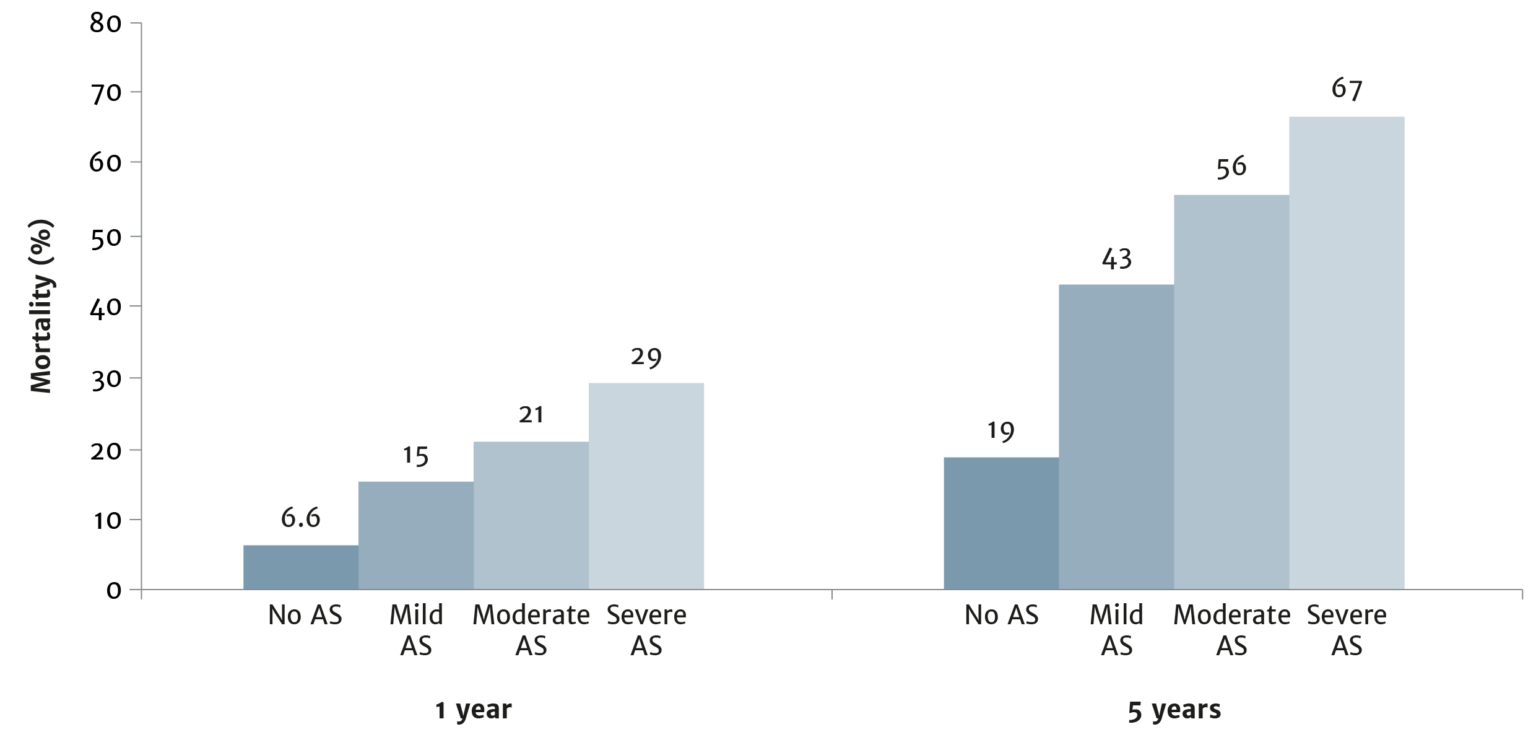
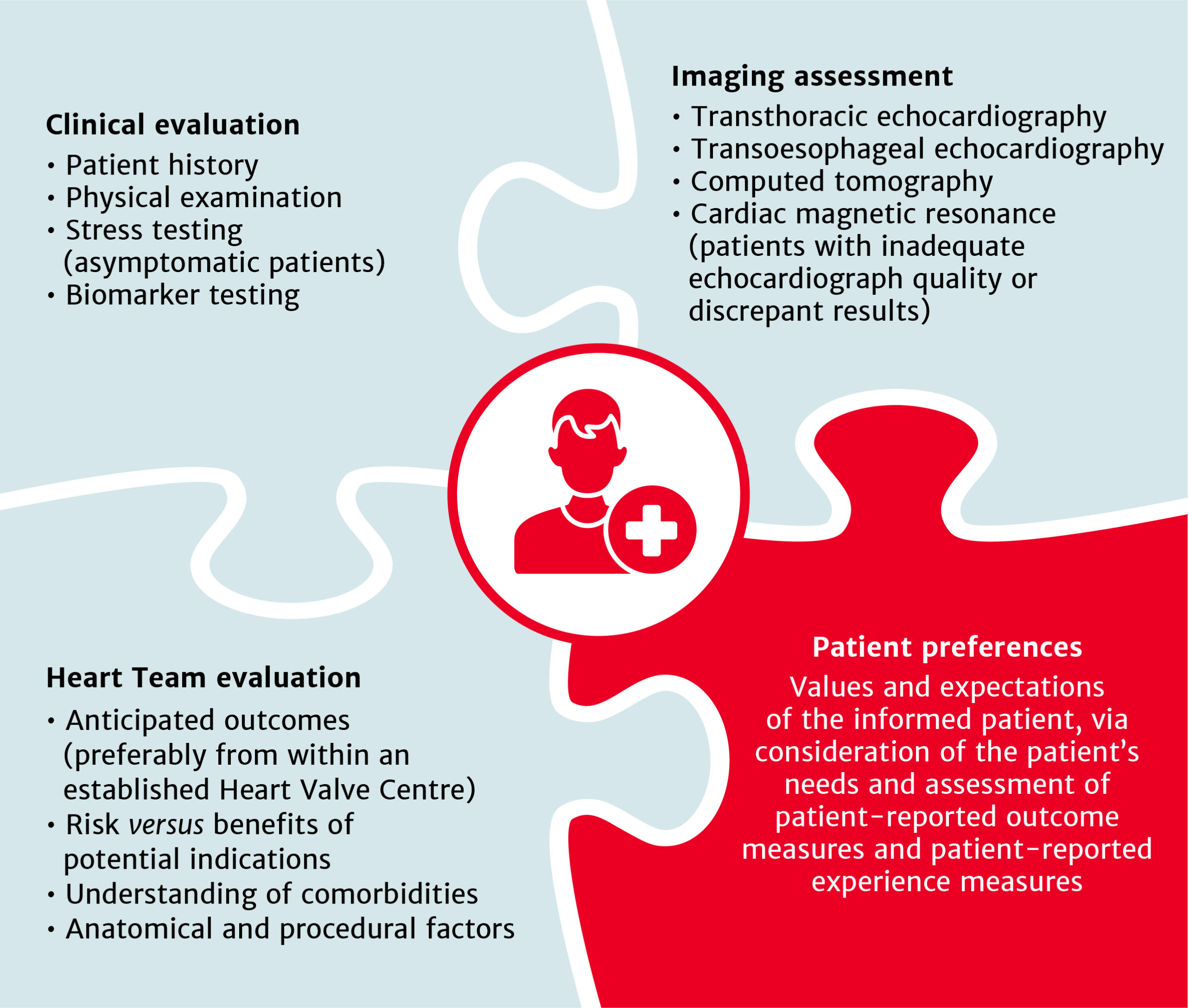
Treatment recommendations for all patients with severe aortic stenosis to be made by the Heart Team
Intervention options for patients with asymptomatic aortic stenosis

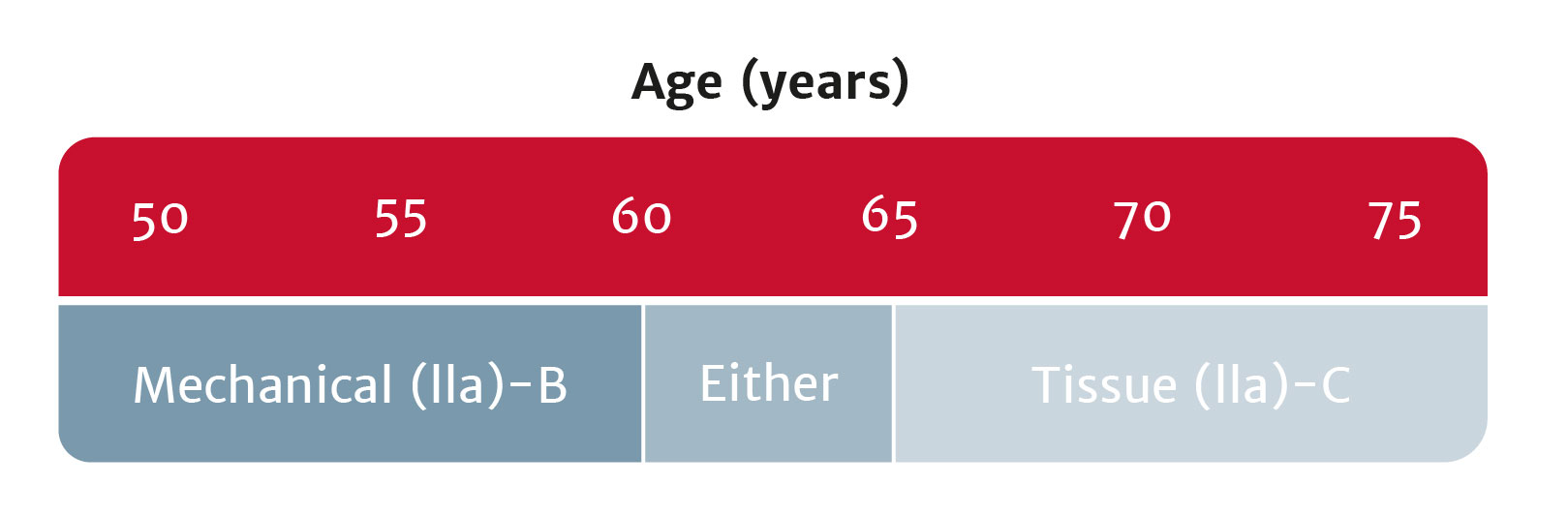
Bioprosthetic valves now recommended for those with life expectancy shorter than valve durability
Intervention recommended for patients with secondary mitral regurgitation, who remain symptomatic despite guideline-directed medical therapy
Use your experience of severe aortic stenosis to ensure the best patient outcomes
The choice between surgical aortic valve replacement (SAVR) and transcatheter aortic valve implantation (TAVI) must be made following evaluation of all factors – clinical, anatomical and procedural – weighing the risks and benefits of each approach for all patients with severe aortic stenosis, and after discussion with the patient.
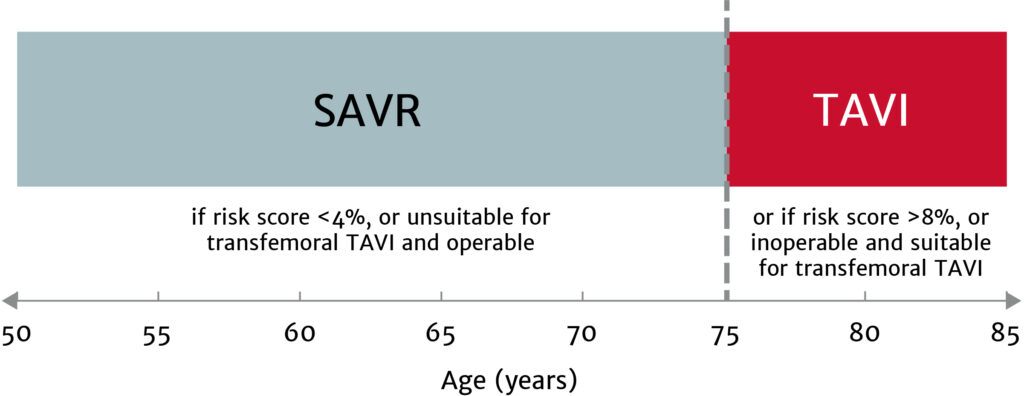
Treatment options have broadened for patients with asymptomatic aortic stenosis, including those with systolic left ventricular (LV) dysfunction (LV ejection fraction <55%) without another cause.
Enable more of your patients to live the active lifestyle they desire
Bioprosthetic valves now have a Class I recommendation for patients with life expectancy lower than the durability of the valve. Now, more of your patients can live a life free from anticoagulation. In addition, bioprosthetic valves may be considered for patients already on long-term novel oral anticoagulants.
Ensure you meet your patients’ high expectations by offering them the best valve technologies.
Be courageous, be experienced, be ethical… according to the guidelines!
Join us from the comfort of your office for a virtual symposium discussing the changes and what they mean for your practice. Eleven speakers from across Europe will cover the implications for the aortic, mitral and tricuspid valves. Click here for the full programme.
Tuesday 21 September 2021
3.00–7.45 pm CET
REGISTER NOW
Coming soon from Edwards
Edwards, Edwards Lifesciences, and the stylized E logo are trademarks or service marks of Edwards Lifesciences Corporation or its affiliates. All other trademarks are the property of their respective owners.
© 2021 Edwards Lifesciences Corporation. All rights reserved. NP–EU-0633 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Dr. Gianluigi Perri
MVT Aortic 2021
MVT Fundamentals Course
MVT Fundamentals Course
MVT Fundamentals Course
Michael Borger, Germany
Jean-Louis Vanoverschelde, Belgium
Can Gollmann-Tepeköylü, Austria
What to know in 2020
Introduction
With almost 380,000 deaths to date and just over 6 million confirmed cases, coronavirus disease 2019 (COVID-19) continues its global spread. In many countries, the first peak in cases has been reached or surpassed, and governments around the world are taking their first tentative steps towards easing lockdown restrictions as they attempt to find the difficult balance between public health and economic priorities.
The scientific effort to tackle the pandemic has brought new insights into the epidemiology and course of the disease, including several new reports detailing the link between COVID-19 and cardiac disease. Multiple studies have been initiated in the search for a vaccine and at least 15 clinical trials are already entering Phase I/II. Additionally, the antiviral remdesivir has become the first drug approved by the FDA for the treatment of COVID-19. Here, we summarise the latest use of cutting-edge science in the fight against COVID-19.
COVID-19 Strategies for impact reduction summary
For professional use.
Edwards Lifesciences, the stylized E logo, are trademarks of Edwards Lifesciences Corporation or its affiliates. All other trademarks or service marks are the property of their respective owners.
© 2020 Edwards Lifesciences Corporation. All rights reserved. PP–EU-0209 v1.0
Edwards Lifesciences• Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Master of Valve Therapy – Nice, February 2019