
November 2, 2023
 ~5m
~5mMeuris B, Roussel JC, Borger MA et […]
Durability of bioprosthetic aortic valve replacement in patients under the age of 60 years – 1-year follow-up from the prospective INDURE registry
Meuris B, Roussel JC, Borger MA et al.
Interdisciplinary CardioVascular and Thoracic Surgery. 2023; doi:10.1093/icvts.ivad115.
Key points:
- The 1-year data from the INSPIRIS RESILIA Durability Registry (INDURE) demonstrate good safety outcomes with surgical aortic valve replacement (SAVR) in young patients characterised by a high rate of bicuspid aortic valve (BAV) and aortic regurgitation; survival rates at 1 year were better than predicted and quality of life (QoL) was fully restored within 3–6 months.
Background information
- Bioprosthetic valves have gained popularity in younger patients due to their durability, decreased reoperation risk and the possibility of transcatheter valve-in-valve procedures, as supported by retrospective studies and current guidelines.1
- The INSPIRIS RESILIA aortic valve, made of bovine pericardial tissue, has shown promising haemodynamic and anticalcification properties in preclinical and clinical studies, including excellent safety and effectiveness at 5 years.2–8 However, data specifically focusing on patients under 60 years of age are limited.
Aim
- To assess 1-year safety and clinical outcomes in patients under 60 years of age undergoing SAVR with the INSPIRIS RESILIA aortic valve.
Type of study
- A prospective, open-label, multicentre international registry with centres across Europe and Canada.
Endpoints
- Primary endpoint:
– Time-related valve safety at 1 year: freedom from specific events according to Valve Academic Research Consortium (VARC)-2 criteria, including repeat procedure, prosthetic valve endocarditis, prosthetic valve thrombosis, thromboembolic events (e.g. stroke) and life-threatening bleeding. - Secondary endpoints:
–Haemodynamic performance assessment.
– Durability evaluation of INSPIRIS RESILIA aortic valve (composite endpoint of time-related valve safety [VARC-2; adjudicated by an independent clinical events committee] and structural valve deterioration [SVD] stage 3 [VARC-3; based on a standardised Core Lab adjudicated assessment]).
– Clinical outcomes: all cause, cardiovascular and valve-related mortality; valve-related dysfunction; need for repeat procedures; permanent pacemaker implantation; acute kidney injury stage 2 or 3; and New York Heart Association (NYHA) functional class.
– Evaluation of QoL using Kansas City Cardiomyopathy Questionnaire (KCCQ) and Short Form-12 Health Survey (SF-12v2).
Methods
- 421 patients who underwent SAVR with the INSPIRIS RESILIA aortic valve at 21 sites across nine countries were included.
- Inclusion criteria included planned native valve replacement based on preoperative evaluation.
- Exclusion criteria included inability to implant valve per device Instructions for Use, active endocarditis/myocarditis within last 3 months, previous aortic valve replacement, Bentall procedure or surgery on other valves, and life expectancy of less than 12 months.
Results
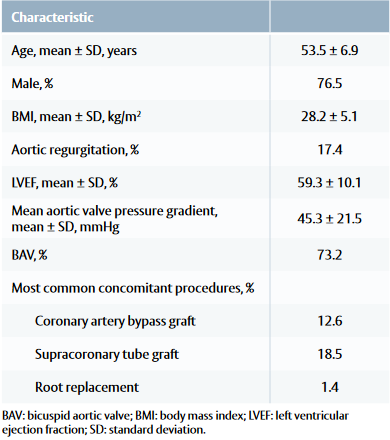
Baseline and procedural characteristic
• Common comorbidities included arterial hypertension, coronary artery disease and type II diabetes.
Table 1: Patient baseline and procedural characteristics
Early (30 days) and 1-year outcomes
- At 30 days, 3 patients (0.7%) died due to cardiovascular causes. No valve-related mortality was reported in the 1-year follow-up.
- Valve-related safety events occurred in 25 patients (5.9%) within 30 days and in 9 patients (2.2% per valve-years) from 30 days to 1 year.
- Within 1 year, 7 patients (1.7%) died, with 5 deaths related to cardiovascular causes. No patients had SVD stage 3 (VARC-3).
- Severe patient–prosthesis mismatch was observed in 1.0% of patients.
- The majority of patients showed improvement in NYHA functional class, with 81.8% in class I and only 3.6% in class III/IV at 1 year, compared with 21.9% and 27.2% at baseline.
- No cases of prosthetic valve leakage were reported.
Quality-of-life outcomes
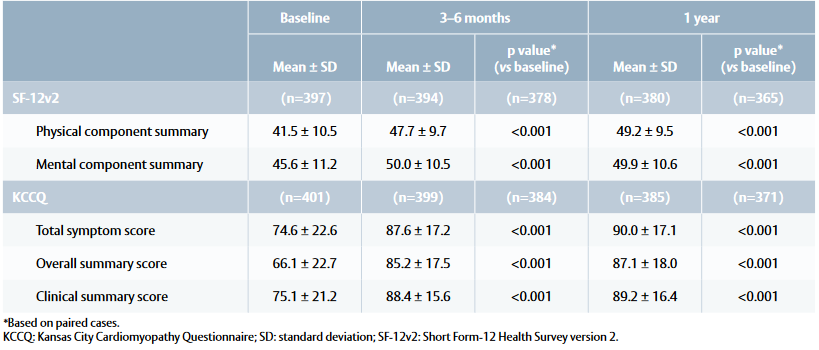
- SF-12v2 physical summary score showed improvements at 3–6 months and 1 year compared with baseline (Table 2). Patients returned to the expected QoL of the general population, achieving a mean score of 50 points within 3–6 months.
- KCCQ total symptom score and clinical summary score increased at 3–6 months and 1 year, with a notable increase in overall summary score at 1 year (Table 2). Patients scored 75–100 points (= good to excellent health status) within 3–6 months.
Table 2: Quality of life outcomes at baseline, 3–6 months and 1 year
Limitations
- The study did not include an active control group, so comparisons between different bioprosthetic valves could not be made and selection bias cannot be excluded. Furthermore, there was no comparison with the outcomes and performance of mechanical valves.
- These results are based on 1-year data, so may not reflect long-term safety outcomes, although the cohort will be followed for 5 years.
Conclusions
- The results of this study demonstrate good outcomes in terms of safety, early improvement in QoL and high survival rates at 1 year for patients under the age of 60 who received the INSPIRIS RESILIA valve.
References
- Christ T, Grubitzsch H, Claus B et al. Stentless aortic valve replacement in the young patient: Long-term results. J Cardiothorac Surg. 2013; 8: 68.
- Flameng W, Hermans H, Verbeken E et al. A randomized assessment of an advanced tissue preservation technology in the juvenile sheep model. J Thorac Cardiovasc Surg. 2015; 149: 340–5.
- Puskas JD, Bavaria JE, Svensson LG et al. The COMMENCE trial: 2-year outcomes with an aortic bioprosthesis with RESILIA tissue. Eur J Cardiothorac Surg. 2017; 52: 432–9.
- Bartus K, Litwinowicz R, Kusmierczyk M et al. Primary safety and effectiveness feasibility study after surgical aortic valve replacement with a new generation bioprosthesis: One-year outcomes. Kardiol Pol. 2018; 76: 618–24.
Bartus K, Litwinowicz R, Bilewska A et al. Intermediate term outcomes after aortic valve replacement with a novel RESILIA™ tissue bioprosthesis. J Thorac Dis. 2019; 11: 3039–46.
Useini D, Schlomicher M, Haldenwang P et al. Early results after aortic valve replacement using last generation bioprosthetic aortic valve. Heart Surg Forum. 2021; 24: E598–962.
Bavaria JE, Griffith B, Heimansohn DA et al. Five year outcomes of the COMMENCE trial investigating aortic valve replacement with RESILIA tissue. Ann Thorac Surg. 2022; 115: 1429–36.
Fukunaga N, Yoshida S, Shimoji A et al. Hemodynamic performance of INSPIRIS RESILIA aortic bioprosthesis for severe aortic stenosis: 2 year follow-up in Japanese cohort. J Artif Organs. 2022; 25: 323–8.
This document is a summary of the Meuris B et al. paper
and covers key information including aim, type of study,
methods, results and conclusion.
No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential
adverse events, please refer to the Instructions for Use (consult eifu.edwards.com where applicable).
Refer to device instructions for use for important warnings related to VFit technology. These features have not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in valve procedures. VFit technology is available on sizes 19–25 mm.
Edwards, Edwards Lifesciences, the stylized E logo, COMMENCE, NSPIRIS, RESILIA, RESILIA and VFit are trademarks or service marks of Edwards Lifesciences Corporation. All other trademarks are the property of their respective owners
© 2023 Edwards Lifesciences Corporation. All rights reserved. PP–EU-6745 v1.0
Edwards Lifesciences • Route de l’Etraz 70, 1260 Nyon, Switzerland • edwards.com
Edwards Masters App
Learn anywhere

now fully customized to your needs and interests. Your educational platform
in heart valve surgery.










