Edwards Lifesciences at the 39th EACTS Annual Meeting
Get the most out of new insights and innovations with
Edwards Lifesciences
Join us for an engaging experience with hands-on training, expert-led sessions, and a showcase of our latest innovations.
Lunch Symposia
A new horizon in aortic root disease management
Explore the latest in aortic root disease management, from surgical innovations like Valsalva vs. straight conduits to the integration of new technologies and pre-assembled valved conduits. Gain practical insights to enhance outcomes in this evolving field.
Chairs:

Ruggero De Paulis, Italy

Joseph Bavaria, USA
Lifetime Management in Light of the 2025 ESC/EACTS Guidelines
Explore how the new guidelines are redefining lifetime management of heart valve disease. This symposium offers practical insights into next-gen bioprosthesis, durable treatment strategies, and real-world case discussions to support long-term patient care.
Chairs:

Christian Carranza, Denmark

Michael Borger, Germany
Training Village: Pioneering Excellence in Cardiac Surgery
The Training Village features six advanced sessions designed to push the boundaries of surgical education—from minimally invasive techniques to transcatheter innovations and complex aortic procedures. It’s a unique opportunity to sharpen your skills, explore emerging technologies, and connect with peers in a hands-on learning environment.
Advanced MIS Skills Lab
This hands-on session focuses on mastering a range of minimally invasive approaches, including trans-axillary access, endoscopic techniques, and mini-thoracotomy. Participants will refine their skills in both aortic and mitral valve procedures through guided simulations designed to enhance precision and efficiency.
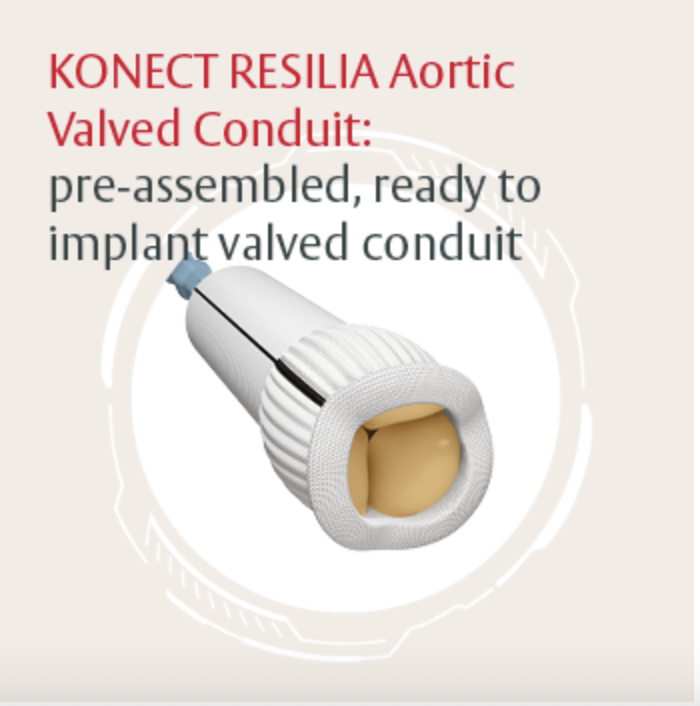
Bentall Procedure with a Novel Valved Conduit
This hands-on session focuses on the Bentall procedure using the KONECT RESILIA valved conduit. Led by experts involved in its development, the session offers practical insights into the device’s design, handling, and clinical use.
Aortic Root Enlargement Wet Lab
Led by the inventor of the technique, this wet lab offers practical training in aortic root enlargement, addressing anatomical challenges and expanding options for patients with small annuli.
THV Applications with the SAPIEN Platform
Explore the newest advancements in transcatheter aortic valve technology and techniques, along with lifetime management strategies.
RESILIA technology
This session offers an overview of RESILIA tissue technology and its clinical applications. Participants will explore key features, durability data, and surgical considerations through interactive discussions and practical demonstrations.
Hands-on experience with EVOQUE TTVR and SAPIEN M3 TMVR system
Join us for an immersive workshop on the latest transcatheter replacement therapies for mitral and tricuspid regurgitation. This interactive session will introduce you to the EVOQUE and SAPIEN M3 systems and will give you the opportunity to take part in a hands-on experience simulating a complete procedure.
Discover Edwards Lifesciences’ latest innovations
Join us at our booth to connect with your peers and discover our products through immersive and engaging experiences, featuring the latest data and studies on aortic and mitral valve surgery.
MITRIS RESILIA valve: Built to handle the pressure of the mitral position
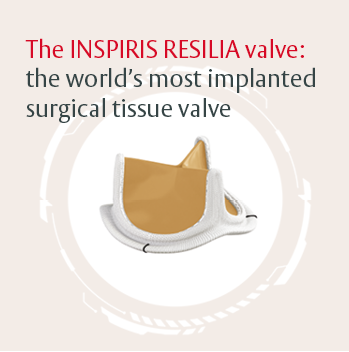
The INSPIRIS RESILIA valve: the world's most implanted surgical tissue valve
Recommended sessions
Thursday 9 Oct 2025
SAVR versus TAVI: More questions than answers?
in the real-world scenario vs randomized clinical trials: the risk of indication creep - Gastino, E.,
From Guidelines to Treatment: Transforming ESC/EACTS Guidelines into Lifelong AS Management
Endoscopic Cardiac Surgery: The Path Less Taken?
The road less traveled: aortic valve insights
Compared to Valve-in-Valve Transcatheter Aortic Valve Implantation
for Degenerated Surgical Bioprostheses - Modbahi, S.,
A subanalysis of the RHEIA-Trial - Bonaros, N.,
2025 EACTS Guidelines Highlights
Friday 10 Oct 2025
Frontiers in minimally invasive mitral valve repair - prediction, sex differences and long-term durability
Late Breaking Clinical Science 2
Presidential Address
Mastering Mitral Disease: Tailored Approaches for Complex Pathologies
After Mitral Valve Replacement with Second-generation Bioprostheses - Soehartono, N.,
Comparative Results From Multi-Center International Registry - Zoni, D.,
Late Breaking Clinical Science 3
Saturday 11 Oct 2025
Expanding indications for TAVI: bicuspid valves and aortic valve regurgitation
Surgery after TAVR implantation
EACTS 2025 Trial Update Session
Following FDA approval, what changes? - Borger, M.,
Where are we heading in revascularisation? - Casselman, F.,

Get Edwards updates
Sign up to our newsletter to stay up-to-date with the latest news and updates.
†Consult instructions for use for device preparation instructions
*Clinical data on valves with RESILIA tissue up to 7-year follow-up have been published, with additional follow-up of up to 10 years in process.
Reference:
1. Beaver T, Bavaria JE, Griffith B, et al. Seven-year outcomes following aortic valve replacement with a novel tissue bioprosthesis. J Thorac Cardiovasc Surg. 2024 Sep;168(3):781-791.
Medical device for professional use. For a listing of indications, contraindications, precautions, warnings, and potential adverse events, please refer to the Instructions for Use (consult https://eifu.edwards.com where applicable).