Explore how the features of the INSPIRIS RESILIA aortic valve might facilitate lifelong management of aortic valve disease.
Patients affected by aortic valve disease seek a long-lasting solution that does not limit their future treatment options or impacts their day-to-day life.1
Valve selection is a lifelong commitment which requires careful consideration of valve durability and the impact it could have on possible secondary interventions.
Recommending the right valve means getting closer to valve therapy and therefore to your patients' needs.
The INSPIRIS RESILIA aortic valve offers your patients a solution that's right for today and ready for tomorrow.
Who could benefit today and tomorrow from the INSPIRIS RESILIA aortic valve?
The INSPIRIS RESILIA aortic valve is suitable for a broad range of patients2 while facilitating the lifetime management of their aortic valve disease.
The 2021 ESC/EACTS guidelines do not recommend bioprostheses in patients below 60 years of age, however, informed patient preference and lifestyle also plays a critical role in the decision-making process for the choice of prosthetic valve.3
The INSPIRIS RESILIA aortic valve is the market-leading replacement aortic valve4
To date, more than 200,000 INSPIRIS RESILIA aortic valves have been implanted into patients worldwide5
Help your patients meet the future confidently, with enhanced anti-calcification tissue and VFit technology designed to allow potential future subsequent valve intervention.2, 6–10
Refer to device instructions for use for important warnings related to VFit technology. These features have not been observed in clinical studies to establish the safety and effectiveness of the model 11500A for use in valve-in-valve procedures. VFit technology is available on sizes 19–25 mm.
Building on a trusted foundation
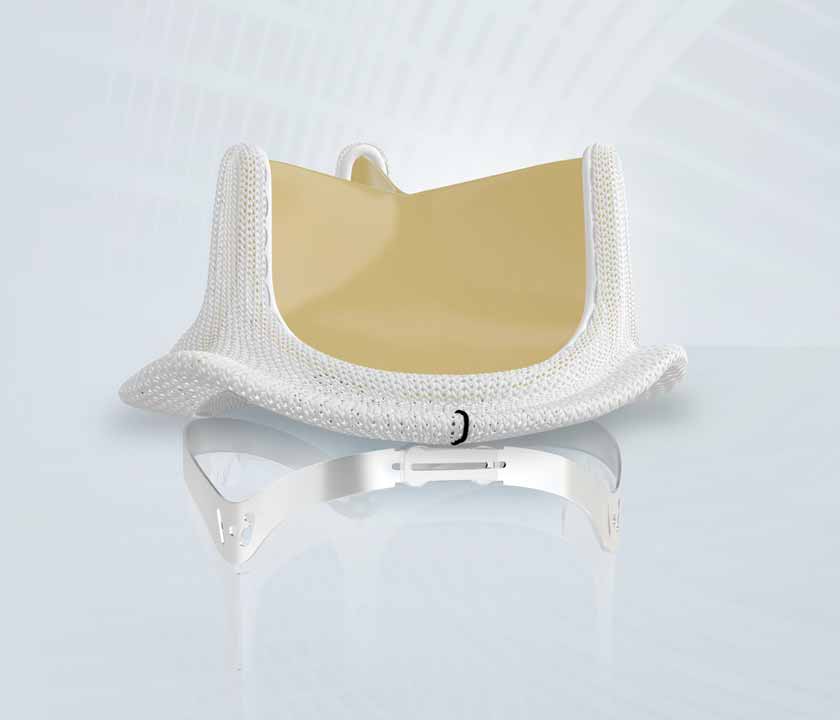
The INSPIRIS RESILIA aortic valve is the latest generation of aortic tissue valve developed by Edwards Lifesciences, and builds upon the proven Carpentier-Edwards PERIMOUNT Magna Ease aortic valve with additional features.
*No clinical data are available that evaluate the long-term impact of the Carpentier–Edwards ThermaFix treatment in patients.
†No clinical data are available that evaluate the long-term impact of RESILIA tissue in patients. The INSPIRIS RESILIA valve alloy band represents 19-25mm sizes. The sizes 27 and 29 mm do not have VFit technology.
The INSPIRIS RESILIA aortic valve can be implanted via minimally invasive procedures17 for faster recovery times.18
Look closer
Right for today, ready for tomorrow.
Sign up now and don't miss any updates about our surgical therapies.
Abbreviations
AS, aortic stenosis; BMI, body-mass index; SAVR, surgical aortic valve replacement; T2D, type 2 diabetes.